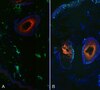
Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination.
Haines, JD; Herbin, O; de la Hera, B; Vidaurre, OG; Moy, GA; Sun, Q; Fung, HY; Albrecht, S; Alexandropoulos, K; McCauley, D; Chook, YM; Kuhlmann, T; Kidd, GJ; Shacham, S; Casaccia, P
Nature neuroscience
18
511-20
2015
Zobrazit abstrakt
Axonal damage has been associated with aberrant protein trafficking. We examined a newly characterized class of compounds that target nucleo-cytoplasmic shuttling by binding to the catalytic groove of the nuclear export protein XPO1 (also known as CRM1, chromosome region maintenance protein 1). Oral administration of reversible CRM1 inhibitors in preclinical murine models of demyelination significantly attenuated disease progression, even when started after the onset of paralysis. Clinical efficacy was associated with decreased proliferation of immune cells, characterized by nuclear accumulation of cell cycle inhibitors, and preservation of cytoskeletal integrity even in demyelinated axons. Neuroprotection was not limited to models of demyelination, but was also observed in another mouse model of axonal damage (that is, kainic acid injection) and detected in cultured neurons after knockdown of Xpo1, the gene encoding CRM1. A proteomic screen for target molecules revealed that CRM1 inhibitors in neurons prevented nuclear export of molecules associated with axonal damage while retaining transcription factors modulating neuroprotection. | | | 25706475
 |
Transplantation of mouse embryonic stem cell-derived oligodendrocytes in the murine model of globoid cell leukodystrophy.
Kuai, XL; Ni, RZ; Zhou, GX; Mao, ZB; Zhang, JF; Yi, N; Liu, ZX; Shao, N; Ni, WK; Wang, ZW
Stem cell research & therapy
6
30
2015
Zobrazit abstrakt
Globoid cell leukodystrophy (GLD) is a severe disorder of the central and peripheral nervous system caused by the absence of galactocerebrosidase (GALC) activity. Cell-based therapies are highly promising strategies for GLD. In this study, G-Olig2 mouse embryonic stem cells (ESCs) were induced into oligodendrocyte progenitor cells (OPCs) and were implanted into the brains of twitcher mice, an animal model of GLD, to explore the therapeutic potential of the cells.The G-Olig2 ESCs were induced into OPCs by using cytokines and a multi-step differentiation procedure. Oligodendrocyte markers were detected by reverse transcription-polymerase chain reaction (RT-PCR) and immunocytochemistry. The toxicity of psychosine to OPCs was determined by a cell proliferation assay kit. The GALC level of OPCs was also examined. OPCs were labeled with Dir and transplanted into the brains of twitcher mice. The transplanted cells were detected by in-Vivo Multispectral Imaging System and real-time PCR. The physiological effects of twitcher mice were assessed.Oligodendrocyte markers were expressed in OPCs, and 76%±5.76% of the OPCs were enhanced green fluorescent protein (eGFP)-positive, eGFP was driven by the Olig2 promoter. The effect of psychosine on cell viability indicated that OPCs were more resistant to psychosine toxicity. The GALC level of OPCs was 10.0±1.23 nmol/hour per mg protein, which was significantly higher than other cells. Dir-labeled OPCs were injected into the forebrain of post-natal day 10 twitcher mice. The transplanted OPCs were myelin basic protein (MBP)-positive and remained along the injection tract as observed by fluorescent microscopy. The level of the Dir fluorescent signal and eGFP mRNA significantly decreased at days 10 and 20 after injection, as indicated by in-Vivo Multispectral Imaging System and real-time PCR. Because of poor cell survival and limited migration ability, there was no significant improvement in brain GALC activity, MBP level, life span, body weight, and behavioral deficits of twitcher mice.ESC-derived OPC transplantation was not sufficient to reverse the clinical course of GLD in twitcher mice. | | | 25888852
 |
Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects.
Cardoso, FL; Herz, J; Fernandes, A; Rocha, J; Sepodes, B; Brito, MA; McGavern, DB; Brites, D
Journal of neuroinflammation
12
82
2015
Zobrazit abstrakt
The inflammatory mediator lipopolysaccharide (LPS) has been shown to induce acute gliosis in neonatal mice. However, the progressive effects on the murine neurodevelopmental program over the week that follows systemic inflammation are not known. Thus, we investigated the effects of repeated LPS administration in the first postnatal week in mice, a condition mimicking sepsis in late preterm infants, on the developing central nervous system (CNS).Systemic inflammation was induced by daily intraperitoneal administration (i.p.) of LPS (6 mg/kg) in newborn mice from postnatal day (PND) 4 to PND6. The effects on neurodevelopment were examined by staining the white matter and neurons with Luxol Fast Blue and Cresyl Violet, respectively. The inflammatory response was assessed by quantifying the expression/activity of matrix metalloproteinases (MMP), toll-like receptor (TLR)-4, high mobility group box (HMGB)-1, and autotaxin (ATX). In addition, B6 CX3CR1(gfp/+) mice combined with cryo-immunofluorescence were used to determine the acute, delayed, and lasting effects on myelination, microglia, and astrocytes.LPS administration led to acute body and brain weight loss as well as overt structural changes in the brain such as cerebellar hypoplasia, neuronal loss/shrinkage, and delayed myelination. The impaired myelination was associated with alterations in the proliferation and differentiation of NG2 progenitor cells early after LPS administration, rather than with excessive phagocytosis by CNS myeloid cells. In addition to disruptions in brain architecture, a robust inflammatory response to LPS was observed. Quantification of inflammatory biomarkers revealed decreased expression of ATX with concurrent increases in HMGB1, TLR-4, and MMP-9 expression levels. Acute astrogliosis (GFAP(+) cells) in the brain parenchyma and at the microvasculature interface together with parenchymal microgliosis (CX3CR1(+) cells) were also observed. These changes preceded the migration/proliferation of CX3CR1(+) cells around the vessels at later time points and the subsequent loss of GFAP(+) astrocytes.Collectively, our study has uncovered a complex innate inflammatory reaction and associated structural changes in the brains of neonatal mice challenged peripherally with LPS. These findings may explain some of the neurobehavioral abnormalities that develop following neonatal sepsis. | | | 25924675
 |
An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer's disease.
Kizuka, Y; Kitazume, S; Fujinawa, R; Saito, T; Iwata, N; Saido, TC; Nakano, M; Yamaguchi, Y; Hashimoto, Y; Staufenbiel, M; Hatsuta, H; Murayama, S; Manya, H; Endo, T; Taniguchi, N
EMBO molecular medicine
7
175-89
2015
Zobrazit abstrakt
The β-site amyloid precursor protein cleaving enzyme-1 (BACE1), an essential protease for the generation of amyloid-β (Aβ) peptide, is a major drug target for Alzheimer's disease (AD). However, there is a concern that inhibiting BACE1 could also affect several physiological functions. Here, we show that BACE1 is modified with bisecting N-acetylglucosamine (GlcNAc), a sugar modification highly expressed in brain, and demonstrate that AD patients have higher levels of bisecting GlcNAc on BACE1. Analysis of knockout mice lacking the biosynthetic enzyme for bisecting GlcNAc, GnT-III (Mgat3), revealed that cleavage of Aβ-precursor protein (APP) by BACE1 is reduced in these mice, resulting in a decrease in Aβ plaques and improved cognitive function. The lack of this modification directs BACE1 to late endosomes/lysosomes where it is less colocalized with APP, leading to accelerated lysosomal degradation. Notably, other BACE1 substrates, CHL1 and contactin-2, are normally cleaved in GnT-III-deficient mice, suggesting that the effect of bisecting GlcNAc on BACE1 is selective to APP. Considering that GnT-III-deficient mice remain healthy, GnT-III may be a novel and promising drug target for AD therapeutics. | | | 25592972
 |
Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination.
da Silva, TF; Eira, J; Lopes, AT; Malheiro, AR; Sousa, V; Luoma, A; Avila, RL; Wanders, RJ; Just, WW; Kirschner, DA; Sousa, MM; Brites, P
The Journal of clinical investigation
124
2560-70
2014
Zobrazit abstrakt
Rhizomelic chondrodysplasia punctata (RCDP) is a developmental disorder characterized by hypotonia, cataracts, abnormal ossification, impaired motor development, and intellectual disability. The underlying etiology of RCDP is a deficiency in the biosynthesis of ether phospholipids, of which plasmalogens are the most abundant form in nervous tissue and myelin; however, the role of plasmalogens in the peripheral nervous system is poorly defined. Here, we used mouse models of RCDP and analyzed the consequence of plasmalogen deficiency in peripheral nerves. We determined that plasmalogens are crucial for Schwann cell development and differentiation and that plasmalogen defects impaired radial sorting, myelination, and myelin structure. Plasmalogen insufficiency resulted in defective protein kinase B (AKT) phosphorylation and subsequent signaling, causing overt activation of glycogen synthase kinase 3β (GSK3β) in nerves of mutant mice. Treatment with GSK3β inhibitors, lithium, or 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8) restored Schwann cell defects, effectively bypassing plasmalogen deficiency. Our results demonstrate the requirement of plasmalogens for the correct and timely differentiation of Schwann cells and for the process of myelination. In addition, these studies identify a mechanism by which the lack of a membrane phospholipid causes neuropathology, implicating plasmalogens as regulators of membrane and cell signaling. | | | 24762439
 |
Progressive disorganization of paranodal junctions and compact myelin due to loss of DCC expression by oligodendrocytes.
Bull, SJ; Bin, JM; Beaumont, E; Boutet, A; Krimpenfort, P; Sadikot, AF; Kennedy, TE
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
9768-78
2014
Zobrazit abstrakt
Paranodal axoglial junctions are critical for maintaining the segregation of axonal domains along myelinated axons; however, the proteins required to organize and maintain this structure are not fully understood. Netrin-1 and its receptor Deleted in Colorectal Cancer (DCC) are proteins enriched at paranodes that are expressed by neurons and oligodendrocytes. To identify the specific function of DCC expressed by oligodendrocytes in vivo, we selectively eliminated DCC from mature myelinating oligodendrocytes using an inducible cre regulated by the proteolipid protein promoter. We demonstrate that DCC deletion results in progressive disruption of the organization of axonal domains, myelin ultrastructure, and myelin protein composition. Conditional DCC knock-out mice develop balance and coordination deficits and exhibit decreased conduction velocity. We conclude that DCC expression by oligodendrocytes is required for the maintenance and stability of myelin in vivo, which is essential for proper signal conduction in the CNS. | | | 25031414
 |
Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons.
Lodato, S; Molyneaux, BJ; Zuccaro, E; Goff, LA; Chen, HH; Yuan, W; Meleski, A; Takahashi, E; Mahony, S; Rinn, JL; Gifford, DK; Arlotta, P
Nature neuroscience
17
1046-54
2014
Zobrazit abstrakt
The neocortex contains an unparalleled diversity of neuronal subtypes, each defined by distinct traits that are developmentally acquired under the control of subtype-specific and pan-neuronal genes. The regulatory logic that orchestrates the expression of these unique combinations of genes is unknown for any class of cortical neuron. Here, we report that Fezf2 is a selector gene able to regulate the expression of gene sets that collectively define mouse corticospinal motor neurons (CSMN). We find that Fezf2 directly induces the glutamatergic identity of CSMN via activation of Vglut1 (Slc17a7) and inhibits a GABAergic fate by repressing transcription of Gad1. In addition, we identify the axon guidance receptor EphB1 as a target of Fezf2 necessary to execute the ipsilateral extension of the corticospinal tract. Our data indicate that co-regulated expression of neuron subtype-specific and pan-neuronal gene batteries by a single transcription factor is one component of the regulatory logic responsible for the establishment of CSMN identity. | Immunocytochemistry | | 24997765
 |
Myelin basic protein induces neuron-specific toxicity by directly damaging the neuronal plasma membrane.
Zhang, J; Sun, X; Zheng, S; Liu, X; Jin, J; Ren, Y; Luo, J
PloS one
9
e108646
2014
Zobrazit abstrakt
The central nervous system (CNS) insults may cause massive demyelination and lead to the release of myelin-associated proteins including its major component myelin basic protein (MBP). MBP is reported to induce glial activation but its effect on neurons is still little known. Here we found that MBP specifically bound to the extracellular surface of the neuronal plasma membrane and induced neurotoxicity in vitro. This effect of MBP on neurons was basicity-dependent because the binding was blocked by acidic lipids and competed by other basic proteins. Further studies revealed that MBP induced damage to neuronal membrane integrity and function by depolarizing the resting membrane potential, increasing the permeability to cations and other molecules, and decreasing the membrane fluidity. At last, artificial liposome vesicle assay showed that MBP directly disturbed acidic lipid bilayer and resulted in increased membrane permeability. These results revealed that MBP induces neurotoxicity through its direct interaction with acidic components on the extracellular surface of neuronal membrane, which may suggest a possible contribution of MBP to the pathogenesis in the CNS disorders with myelin damage. | | | 25255088
 |
Glial ankyrins facilitate paranodal axoglial junction assembly.
Chang, KJ; Zollinger, DR; Susuki, K; Sherman, DL; Makara, MA; Brophy, PJ; Cooper, EC; Bennett, V; Mohler, PJ; Rasband, MN
Nature neuroscience
17
1673-81
2014
Zobrazit abstrakt
Neuron-glia interactions establish functional membrane domains along myelinated axons. These include nodes of Ranvier, paranodal axoglial junctions and juxtaparanodes. Paranodal junctions are the largest vertebrate junctional adhesion complex, and they are essential for rapid saltatory conduction and contribute to assembly and maintenance of nodes. However, the molecular mechanisms underlying paranodal junction assembly are poorly understood. Ankyrins are cytoskeletal scaffolds traditionally associated with Na(+) channel clustering in neurons and are important for membrane domain establishment and maintenance in many cell types. Here we show that ankyrin-B, expressed by Schwann cells, and ankyrin-G, expressed by oligodendrocytes, are highly enriched at the glial side of paranodal junctions where they interact with the essential glial junctional component neurofascin 155. Conditional knockout of ankyrins in oligodendrocytes disrupts paranodal junction assembly and delays nerve conduction during early development in mice. Thus, glial ankyrins function as major scaffolds that facilitate early and efficient paranodal junction assembly in the developing CNS. | | | 25362471
 |
Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep.
Kuypers, E; Jellema, RK; Ophelders, DR; Dudink, J; Nikiforou, M; Wolfs, TG; Nitsos, I; Pillow, JJ; Polglase, GR; Kemp, MW; Saito, M; Newnham, JP; Jobe, AH; Kallapur, SG; Kramer, BW
PloS one
8
e81644
2013
Zobrazit abstrakt
Chorioamnionitis and antenatal glucocorticoids are common exposures for preterm infants and can affect the fetal brain, contributing to cognitive and motor deficits in preterm infants. The effects of antenatal glucocorticoids on the brain in the setting of chorioamnionitis are unknown. We hypothesized that antenatal glucocorticoids would modulate inflammation in the brain and prevent hippocampal and white matter injury after intra-amniotic lipopolysaccharide (LPS) exposure.Time-mated ewes received saline (control), an intra-amniotic injection of 10 mg LPS at 106d GA or 113d GA, maternal intra-muscular betamethasone (0.5 mg/kg maternal weight) alone at 113d GA, betamethasone at 106d GA before LPS or betamethasone at 113d GA after LPS. Animals were delivered at 120d GA (term=150d). Brain structure volumes were measured on T2-weighted MRI images. The subcortical white matter (SCWM), periventricular white matter (PVWM) and hippocampus were analyzed for microglia, astrocytes, apoptosis, proliferation, myelin and pre-synaptic vesicles.LPS and/or betamethasone exposure at different time-points during gestation did not alter brain structure volumes on MRI. Betamethasone alone did not alter any of the measurements. Intra-amniotic LPS at 106d or 113d GA induced inflammation as indicated by increased microglial and astrocyte recruitment which was paralleled by increased apoptosis and hypomyelination in the SCWM and decreased synaptophysin density in the hippocampus. Betamethasone before the LPS exposure at 113d GA prevented microglial activation and the decrease in synaptophysin. Betamethasone after LPS exposure increased microglial infiltration and apoptosis.Intra-uterine LPS exposure for 7d or 14d before delivery induced inflammation and injury in the fetal white matter and hippocampus. Antenatal glucocorticoids aggravated the inflammatory changes in the brain caused by pre-existing intra-amniotic inflammation. Antenatal glucocorticoids prior to LPS reduced the effects of intra-uterine inflammation on the brain. The timing of glucocorticoid administration in the setting of chorioamnionitis can alter outcomes for the fetal brain. | | | 24358119
 |