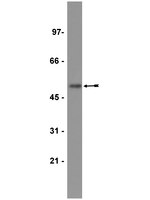
The L84F polymorphic variant of human O6-methylguanine-DNA methyltransferase alters stability in U87MG glioma cells but not temozolomide sensitivity.
Remington, M; Chtchetinin, J; Ancheta, K; Nghiemphu, PL; Cloughesy, T; Lai, A
Neuro-oncology
11
22-32
2009
Zobrazit abstrakt
First-line therapy for patients with glioblastoma multiforme includes treatment with radiation and temozolomide (TMZ), an oral DNA alkylating chemotherapy. Sensitivity of glioma cells to TMZ is dependent on the level of cellular O(6)-methylguanine-DNA methyltransferase (MGMT) repair activity. Several common coding-region polymorphisms in the MGMT gene (L84F and the linked pair I143V/K178R) modify functional characteristics of MGMT and cancer risk. To determine whether these polymorphic changes influence the ability of MGMT to protect glioma cells from TMZ, we stably overexpressed enhanced green fluorescent protein (eGFP)-tagged MGMT constructs in U87MG glioma cells. We confirmed that the wild-type (WT) eGFP-MGMT protein is properly localized within the nucleus and found that L84F, I143V/K178R, and L84F/I143V/K178R eGFP-MGMT variants exhibited nuclear localization patterns indistinguishable from WT. Using MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] proliferation and clonogenic survival assays, we confirmed that WT cells expressing eGFP-MGMT are resistant to TMZ treatment compared with control U87MG cells, and that each of the polymorphic eGFP-MGMT variants confers similar resistance to TMZ. However, upon exposure to O(6)-benzylguanine (O(6)-BG), a synthetic MGMT inhibitor, the L84F and L84F/I143V/K178R variants were degraded more rapidly than WT or I143V/K178R in a proteasome-dependent manner. Despite the increased O(6)-BG- stimulated protein turnover caused by the L84F alteration, cells expressing L84F eGFP-MGMT did not exhibit altered sensitivity to the combination of O(6)-BG and TMZ compared with WT cells. In conclusion, we demonstrated that the L84F polymorphic variant has altered protein turnover without modifying sensitivity of U87MG cells to TMZ or combined TMZ and O(6)-BG. These findings may provide a clue to determining the clinical significance of MGMT coding-region polymorphisms. | Western Blotting | 18812520
 |
Cation exchange chromatography in antibody purification: pH screening for optimised binding and HCP removal.
Andreas Stein,André Kiesewetter
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences
848
2007
Zobrazit abstrakt
The production of pharmaceutical antibodies requires reliable and rapid processes with high purity and yield. Although protein A gels selectively and efficiently bind antibodies in the capture step, intense research is going on to find alternatives that can abolish the drawbacks of protein A chromatography. Ion exchangers e.g. are more robust, considerably cheaper and can eliminate ligand leaching. For the strong cation exchangers Fractogel EMD SO3- (M) and Fractogel EMD SE Hicap (M) we have evaluated the influence of pH for optimised binding and removal of host cell protein (HCP). In a fast initial screening we measured batch binding capacities. Subsequent scale-down to 96-well plate format proved that assay miniaturisation still provided reliable data. We demonstrated with the principle of residence time that scout columns are suitable for dynamic studies. The optimum pH range from batch binding was transferred to scout columns which were then used to screen for maximum dynamic capacities. In addition IEF titration curve analysis was employed to define a final operational pH. With this pH we ran labscale columns to purify monoclonal antibody. The cation exchangers showed high step yields and host cell proteins in the pools from gradient elution were reduced very effectively. | | 17113367
 |
Cloning and characterization of GDP-perosamine synthetase (Per) from Escherichia coli O157:H7 and synthesis of GDP-perosamine in vitro.
Guohui Zhao,Jun Liu,Xiang Liu,Min Chen,Houcheng Zhang,Peng George Wang
Biochemical and biophysical research communications
363
2007
Zobrazit abstrakt
GDP-perosamine synthetase (Per, E.C. not yet classified) is important to the synthesis of Escherichia coli O157:H7 O-antigen. The mutant in per gene can disrupt the synthesis of O157 O-antigen. In this study, GDP-perosamine synthetase was cloned from E. coli O157:H7 and over-expressed in E. coli BL21 (DE3). The recombinant His-tagged Per fusion protein was a decamer with molecular weight of 431 kDa. The optimal pH value of this recombinant protein was 7.5. The divalent ions had no significant effect on Per-catalyzed reaction. The K(m) and K(cat)/K(m) for GDP-4-keto-6-deoxy-d-mannose were 0.09 mM and 2.1 x 10(5)M(-1)S(-1), and those for l-glutamate were 2mM and 0.52 x 10(5)M(-1)S(-1), respectively. Per was used to synthesize GDP-perosamine from GDP-mannose together with recombinant GDP-mannose dehydratase (GMD, E.C. 4.2.1.47). The purified GDP-perosamine was identified by MS and NMR. In summary, this work provided a feasible approach for the synthesis of GDP-perosamine which can lead to the study of LPS biosynthesis of pathogenic E. coli O157:H7. | | 17888872
 |
Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes.
Robson, K J, et al.
EMBO J., 14: 3883-94 (1995)
1994
Zobrazit abstrakt
Plasmodium sporozoites collected from oocysts, haemocoel and salivary glands of the mosquito show profound differences in their biological properties such as motility, ability to induce protective immune response and infectivity for vertebrate host cells. Sporozoites from salivary glands are much more infectious than those from oocysts and haemocoel. Differential expression of proteins, such as the circumsporozoite (CS) protein and the thrombospondin-related adhesive protein (TRAP), implicated in sporozoite recognition and entry into hepatocytes may account for the development of infectivity during ontogeny. We have carried out a series of experiments to: (i) analyse the expression and localization of TRAP in P.falciparum sporozoites during development in the mosquito; and (ii) elucidate the biochemical and adhesive properties of recombinant TRAP. Our data indicate that TRAP is not expressed in oocysts, whereas variable amounts of CS protein are found in this parasite developmental stage. Hemocoel sporozoites display the distinct phenotypes TRAP- CS protein+ and TRAP+ CS protein+ at a frequency of 98.5 and 1.5% respectively. Salivary gland sporozoites are all TRAP+ CS protein+. We also provide experimental evidence showing that recombinant TRAP binds to the basolateral cell membrane of hepatocytes in the Disse's space and that sulfated glycoconjugates function as TRAP ligands on human hepatocytes. | | 7664729
 |