Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina.
Puthussery, T; Percival, KA; Venkataramani, S; Gayet-Primo, J; Grünert, U; Taylor, WR
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
7611-21
2014
Zobrazit abstrakt
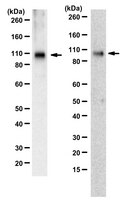
Visual signals are segregated into parallel pathways at the first synapse in the retina between cones and bipolar cells. Within the OFF pathways of mammals, the selective expression of AMPA or kainate-type glutamate receptors in the dendrites of different OFF-bipolar cell types is thought to contribute to formation of distinct temporal channels. AMPA receptors, with rapid recovery from desensitization, are proposed to transmit high temporal frequency signals, whereas kainate receptors (KARs) are presumed to encode lower temporal frequencies. Here we studied the glutamate receptors expressed by OFF-bipolar cells in slice preparations of macaque monkey retina, where the low (midget/parvocellular) and high-frequency (parasol/magnocellular) temporal channels are well characterized. We found that all OFF-bipolar types receive input primarily through KARs and that KAR antagonists block light-evoked input to both OFF-midget and OFF-parasol ganglion cells. KAR subunits were differentially expressed in OFF-bipolar types; the diffuse bipolar (DB) cells, DB2 and DB3b, expressed GluK1 and showed transient responses to glutamate and the KAR agonist, ATPA. In contrast, flat midget bipolar, DB1, and DB3a cells lacked GluK1 and showed relatively sustained responses. Finally, we found that the KAR accessory protein, Neto1, is expressed at the base of cone pedicles but is not colocalized with the GluK1 subunit. In summary, the results indicate that transient signaling in the OFF pathway of macaques is not dependent on AMPA receptors and that heterogeneity of KARs and accessory proteins may contribute to the formation of parallel temporal channels. | Immunohistochemistry | | 24872565
 |
Neuronal pentraxin 2 supports clear cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4.
von Roemeling, CA; Radisky, DC; Marlow, LA; Cooper, SJ; Grebe, SK; Anastasiadis, PZ; Tun, HW; Copland, JA
Cancer research
74
4796-810
2014
Zobrazit abstrakt
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of kidney cancer and has the highest propensity to manifest as metastatic disease. Recent characterizations of the genetic signature of ccRCC have revealed several factors correlated with tumor cell migration and invasion; however, the specific events driving malignancy are not well defined. Furthermore, there remains a lack of targeted therapies that result in long-term, sustainable response in patients with metastatic disease. We show here that neuronal pentraxin 2 (NPTX2) is overexpressed specifically in ccRCC primary tumors and metastases, and that it contributes to tumor cell viability and promotes cell migration through its interaction with the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit GluR4. We propose NPTX2 as a novel molecular target for therapy for patients with ccRCC diagnosed with or at risk of developing metastatic disease. | | | 24962026
 |
Pain after discontinuation of morphine treatment is associated with synaptic increase of GluA4-containing AMPAR in the dorsal horn of the spinal cord.
Cabañero, D; Baker, A; Zhou, S; Hargett, GL; Irie, T; Xia, Y; Beaudry, H; Gendron, L; Melyan, Z; Carlton, SM; Morón, JA
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology
38
1472-84
2013
Zobrazit abstrakt
Withdrawal from prescribed opioids results in increased pain sensitivity, which prolongs the treatment. This pain sensitivity is attributed to neuroplastic changes that converge at the spinal cord dorsal horn. We have recently reported that repeated morphine administration triggers an insertion of GluA2-lacking (Ca(2+)-permeable) α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAR) in the hippocampus. This finding together with the reported involvement of AMPAR in the mechanisms underlying inflammatory pain led us to hypothesize a role for spinal AMPAR in opioid-induced pain behavior. Mice treated with escalating doses of morphine showed hypersensitivity to mechanical stimulation. Intrathecal administration of a Ca(2+)-permeable AMPAR selective blocker disrupted morphine-induced mechanical sensitivity. Analysis of the expression and phosphorylation levels of AMPAR subunits (GluA1/2/3/4) in homogenates and in postsynaptic density fractions from spinal cord dorsal horns showed an increase in GluA4 expression and phosphorylation in the postsynaptic density after morphine. Co-immunoprecipitation analyses suggested an increase in GluA4 homomers (Ca(2+)-permeable AMPAR) and immunohistochemical staining localized the increase in GluA4 levels in laminae III-V. The excitatory postsynaptic currents (EPSCs) recorded in laminae III-V showed enhanced sensitivity to Ca(2+)-permeable AMPAR blockers in morphine-treated mice. Furthermore, current-voltage relationships of AMPAR-mediated EPSCs showed that rectification index (an indicator of Ca(2+)-permeable AMPAR contribution) is increased in morphine-treated but not in saline-treated mice. These effects could be reversed by infusion of GluA4 antibody through patch pipette. This is the first direct evidence for a role of GluA4-containing AMPAR in morphine-induced pain and highlights spinal GluA4-containing AMPAR as targets to prevent the morphine-induced pain sensitivity. | Western Blotting | Mouse | 23403695
 |
Evidence for glutamate as a neuroglial transmitter within sensory ganglia.
Kung, LH; Gong, K; Adedoyin, M; Ng, J; Bhargava, A; Ohara, PT; Jasmin, L
PloS one
8
e68312
2013
Zobrazit abstrakt
This study examines key elements of glutamatergic transmission within sensory ganglia of the rat. We show that the soma of primary sensory neurons release glutamate when depolarized. Using acute dissociated mixed neuronal/glia cultures of dorsal root ganglia (DRG) or trigeminal ganglia and a colorimetric assay, we show that when glutamate uptake by satellite glial cells (SGCs) is inhibited, KCl stimulation leads to simultaneous increase of glutamate in the culture medium. With calcium imaging we see that the soma of primary sensory neurons and SGCs respond to AMPA, NMDA, kainate and mGluR agonists, and selective antagonists block this response. Using whole cell patch-clamp technique, inward currents were recorded from small diameter (less than 30 µm) DRG neurons from intact DRGs (ex-vivo whole ganglion preparation) in response to local application of the above glutamate receptor agonists. Following a chronic constriction injury (CCI) of either the inferior orbital nerve or the sciatic nerve, glutamate expression increases in the trigeminal ganglia and DRG respectively. This increase occurs in neurons of all diameters and is present in the somata of neurons with injured axons as well as in somata of neighboring uninjured neurons. These data provides additional evidence that glutamate can be released within the sensory ganglion, and that the somata of primary sensory neurons as well as SGCs express functional glutamate receptors at their surface. These findings, together with our previous gene knockdown data, suggest that glutamatergic transmission within the ganglion could impact nociceptive threshold. | Immunohistochemistry | Rat | 23844184
 |
Histamine receptors of cones and horizontal cells in Old World monkey retinas.
Vila, A; Satoh, H; Rangel, C; Mills, SL; Hoshi, H; O'Brien, J; Marshak, DR; Macleish, PR; Marshak, DW
The Journal of comparative neurology
520
528-43
2011
Zobrazit abstrakt
In primates the retina receives input from histaminergic neurons in the posterior hypothalamus that are active during the day. In order to understand how this input contributes to information processing in Old World monkey retinas, we have been localizing histamine receptors (HR) and studying the effects of histamine on the neurons that express them. Previously, we localized HR3 to the tips of ON bipolar cell dendrites and showed that histamine hyperpolarizes the cells via this receptor. We raised antisera against synthetic peptides corresponding to an extracellular domain of HR1 between the 4th and 5th transmembrane domains and to an intracellular domain near the carboxyl terminus of HR2. Using these, we localized HR1 to horizontal cells and a small number of amacrine cells and localized HR2 to puncta closely associated with synaptic ribbons inside cone pedicles. Consistent with this, HR1 mRNA was detected in horizontal cell perikarya and primary dendrites and HR2 mRNA was found in cone inner segments. We studied the effect of 5 μM exogenous histamine on primate cones in macaque retinal slices. Histamine reduced I(h) at moderately hyperpolarized potentials, but not the maximal current. This would be expected to increase the operating range of cones and conserve ATP in bright, ambient light. Thus, all three major targets of histamine are in the outer plexiform layer, but the retinopetal axons containing histamine terminate in the inner plexiform layer. Taken together, the findings in these three studies suggest that histamine acts primarily via volume transmission in primate retina. | | | 21800315
 |
Amacrine and bipolar inputs to midget and parasol ganglion cells in marmoset retina.
Carla J Abbott,Kumiko A Percival,Paul R Martin,Ulrike Grünert
Visual neuroscience
29
2011
Zobrazit abstrakt
Retinal ganglion cells receive excitatory synapses from bipolar cells and inhibitory synapses from amacrine cells. Previous studies in primate suggest that the strength of inhibitory amacrine input is greater to cells in peripheral retina than to foveal (central) cells. A comprehensive study of a large number of ganglion cells at different eccentricities, however, is still lacking. Here, we compared the amacrine and bipolar input to midget and parasol ganglion cells in central and peripheral retina of marmosets (Callithrix jacchus). Ganglion cells were labeled by retrograde filling from the lateral geniculate nucleus or by intracellular injection. Presumed amacrine input was identified with antibodies against gephyrin; presumed bipolar input was identified with antibodies against the GluR4 subunit of the AMPA receptor. In vertical sections, about 40% of gephyrin immunoreactive (IR) puncta were colocalized with GABAA receptor subunits, whereas immunoreactivity for gephyrin and GluR4 was found at distinct sets of puncta. The density of gephyrin IR puncta associated with ganglion cell dendrites was comparable for midget and parasol cells at all eccentricities studied (up to 2 mm or about 16 degrees of visual angle for midget cells and up to 10 mm or >80 degrees of visual angle for parasol cells). In central retina, the densities of gephyrin IR and GluR4 IR puncta associated with the dendrites of midget and parasol cells are comparable, but the average density of GluR4 IR puncta decreased slightly in peripheral parasol cells. These anatomical results indicate that the ratio of amacrine to bipolar input does not account for the distinct functional properties of parasol and midget cells or for functional differences between cells of the same type in central and peripheral retina. | | | 22564345
 |
Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions.
Reimers, JM; Milovanovic, M; Wolf, ME
Brain research
1367
223-33
2010
Zobrazit abstrakt
The subunit composition of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) is an important determinant of AMPAR biophysical properties and trafficking. To date, AMPAR subunit composition has been quantitatively evaluated only for the hippocampus, where different experimental approaches have yielded different results. Here, we used quantitative co-immunoprecipitation to characterize GluA1-3 associations in the adult rat nucleus accumbens, dorsal striatum, prefrontal cortex, and hippocampus, and blue native electrophoresis (BNE) to study GluA1-3 assembly state. In all brain regions, co-immunoprecipitation experiments showed that ~90% of GluA1 was associated with GluA2 or GluA3 (most was GluA1A2). All regions contained a small number of GluA1A3 receptors. Homomeric GluA1 receptors may also exist. More than half of the GluA2 (53%-65% depending on the region) was not associated with GluA1. However, this represents an over-estimate of the percent of GluA2 present in GluA2A3 receptors, based on BNE results demonstrating that the majority of GluA2 exists as dimers, rather than functional tetrameric receptors. Relatively more GluA1 was present in tetramers. Together with other findings, our results suggest a dominant role for GluA1A2 receptors in all brain regions examined. They also help explain why different results for hippocampal AMPAR subunit composition were obtained using co-immunoprecipitation, which assesses the total cellular pool of AMPARs including partially assembled AMPARs in intracellular compartments, and electrophysiological approaches, which can selectively assess tetrameric (functional) AMPARs on the cell surface. | Immunoprecipitation | | 20946890
 |
Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells.
Zonouzi, M; Renzi, M; Farrant, M; Cull-Candy, SG
Nature neuroscience
14
1430-8
2010
Zobrazit abstrakt
Oligodendrocyte precursor cells (OPCs), a major glial cell type that gives rise to myelinating oligodendrocytes in the CNS, express calcium-permeable AMPA receptors (CP-AMPARs). Although CP-AMPARs are important for OPC proliferation and neuron-glia signaling, they render OPCs susceptible to ischemic damage in early development. We identified factors controlling the dynamic regulation of AMPAR subtypes in OPCs from rat optic nerve and mouse cerebellar cortex. We found that activation of group 1 mGluRs drove an increase in the proportion of CP-AMPARs, reflected by an increase in single-channel conductance and inward rectification. This plasticity required the elevation of intracellular calcium and used PI3K, PICK-1 and the JNK pathway. In white matter, neurons and astrocytes release both ATP and glutamate. Unexpectedly, activation of purinergic receptors in OPCs decreased CP-AMPAR expression, suggesting a capacity for homeostatic regulation. Finally, we found that stargazin-related transmembrane AMPAR regulatory proteins, which are critical for AMPAR surface expression in neurons, regulate CP-AMPAR plasticity in OPCs. | Western Blotting | | 21983683
 |
Immunohistochemical identification and synaptic inputs to the diffuse bipolar cell type DB1 in macaque retina.
Puthussery, T; Gayet-Primo, J; Taylor, WR; Haverkamp, S
The Journal of comparative neurology
519
3640-56
2010
Zobrazit abstrakt
Detailed analysis of the synaptic inputs to the primate DB1 bipolar cell has been precluded by the absence of a suitable immunohistochemical marker. Here we demonstrate that antibodies for the EF-hand calcium-binding protein, secretagogin, strongly label the DB1 bipolar cell as well as a mixed population of GABAergic amacrine cells in the macaque retina. Using secretagogin as a marker, we show that the DB1 bipolar makes synaptic contact with both L/M as well as S-cone photoreceptors and only minimal contact with rod photoreceptors. Electron microscopy showed that the DB1 bipolar makes flat contacts at both triad-associated and nontriad-associated positions on the cone pedicle. Double labeling with various glutamate receptor subunit antibodies failed to conclusively determine the subunit composition of the glutamate receptors on DB1 bipolar cells. In the IPL, DB1 bipolar cell axon terminals expressed the glycine receptor, GlyRα1, at sites of contact with AII amacrine cells, suggesting that these cells receive input from the rod pathway. | | | 22006647
 |
Cell-type-specific localization of protocadherin β16 at AMPA and AMPAKainate receptor-containing synapses in the primate retina.
Puller C, Haverkamp S
J Comp Neurol
519
467-79.
2010
Zobrazit abstrakt
Protocadherins (Pcdhs) are thought to be key features of cell-type-specific synapse formation. Here we analyzed the expression pattern of Pcdh subunit β16 (β16) in the primate retina by applying antibodies against β16, different subunits of ionotropic glutamate receptors (GluRs), and cell-type-specific markers as well as by coimmunoprecipitation and Western blots. Immunocytochemical localization was analyzed by confocal microscopy and preembedding electron microscopy. In the outer plexiform layer (OPL) H1, but not H2, horizontal cells expressed β16 as revealed by the strong reduction of β16 at short-wavelength-sensitive cones. β16 colocalized with the GluR subunits GluR2-4 at horizontal cell dendritic tips and with GluR2-4 and GluR6/7 at the desmosome-like junctions. At the latter, these AMPA and kainate receptor subunits were found to be clustered within single synaptic hot spots. Additionally, β16-labeled dendritic tips of OFF cone bipolar cells appeared in triad-associated positions at the cone pedicle base, pointing to β16 expression by OFF midget or DB3 bipolar cells. In the inner plexiform layer, β16 was localized also postsynaptically at most of the glutamatergic synapses. Overall, we provide evidence for a cell-type-specific localization of β16 together with GluRs at defined postsynaptic sites and a coexistence of AMPA and kainate receptors within single synaptic hot spots. This study supports the hypothesis that β16 plays an important role in the formation and/or stabilization of specific glutamatergic synapses, whereas our in vivo protein biochemical results argue against the existence of protein complexes formed by β16 and GluRs.© 2010 Wiley-Liss, Inc. | | | 21192079
 |