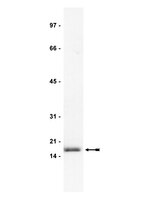
High molecular weight fibroblast growth factor-2 in the human heart is a potential target for prevention of cardiac remodeling.
Santiago, JJ; McNaughton, LJ; Koleini, N; Ma, X; Bestvater, B; Nickel, BE; Fandrich, RR; Wigle, JT; Freed, DH; Arora, RC; Kardami, E
PloS one
9
e97281
2014
Zobrazit abstrakt
Fibroblast growth factor 2 (FGF-2) is a multifunctional protein synthesized as high (Hi-) and low (Lo-) molecular weight isoforms. Studies using rodent models showed that Hi- and Lo-FGF-2 exert distinct biological activities: after myocardial infarction, rat Lo-FGF-2, but not Hi-FGF-2, promoted sustained cardioprotection and angiogenesis, while Hi-FGF-2, but not Lo-FGF-2, promoted myocardial hypertrophy and reduced contractile function. Because there is no information regarding Hi-FGF-2 in human myocardium, we undertook to investigate expression, regulation, secretion and potential tissue remodeling-associated activities of human cardiac (atrial) Hi-FGF-2. Human patient-derived atrial tissue extracts, as well as pericardial fluid, contained Hi-FGF-2 isoforms, comprising, respectively, 53%(±20 SD) and 68% (±25 SD) of total FGF-2, assessed by western blotting. Human atrial tissue-derived primary myofibroblasts (hMFs) expressed and secreted predominantly Hi-FGF-2, at about 80% of total. Angiotensin II (Ang II) up-regulated Hi-FGF-2 in hMFs, via activation of both type 1 and type 2 Ang II receptors; the ERK pathway; and matrix metalloprotease-2. Treatment of hMFs with neutralizing antibodies selective for human Hi-FGF-2 (neu-AbHi-FGF-2) reduced accumulation of proteins associated with fibroblast-to-myofibroblast conversion and fibrosis, including α-smooth muscle actin, extra-domain A fibronectin, and procollagen. Stimulation of hMFs with recombinant human Hi-FGF-2 was significantly more potent than Lo-FGF-2 in upregulating inflammation-associated proteins such as pro-interleukin-1β and plasminogen-activator-inhibitor-1. Culture media conditioned by hMFs promoted cardiomyocyte hypertrophy, an effect that was prevented by neu-AbHi-FGF-2 in vitro. In conclusion, we have documented that Hi-FGF-2 represents a substantial fraction of FGF-2 in human cardiac (atrial) tissue and in pericardial fluid, and have shown that human Hi-FGF-2, unlike Lo-FGF-2, promotes deleterious (pro-fibrotic, pro-inflammatory, and pro-hypertrophic) responses in vitro. Selective targeting of Hi-FGF-2 production may, therefore, reduce pathological remodelling in the human heart. | Western Blotting | 24827991
 |
EphA2-induced angiogenesis in ewing sarcoma cells works through bFGF production and is dependent on caveolin-1.
Sáinz-Jaspeado, M; Huertas-Martinez, J; Lagares-Tena, L; Martin Liberal, J; Mateo-Lozano, S; de Alava, E; de Torres, C; Mora, J; Del Muro, XG; Tirado, OM
PloS one
8
e71449
2013
Zobrazit abstrakt
Angiogenesis is the result of the combined activity of the tumor microenvironment and signaling molecules. The angiogenic switch is represented as an imbalance between pro- and anti-angiogenic factors and is a rate-limiting step in the development of tumors. Eph receptor tyrosine kinases and their membrane-anchored ligands, known as ephrins, constitute the largest receptor tyrosine kinase (RTK) subfamily and are considered a major family of pro-angiogenic RTKs. Ewing sarcoma (EWS) is a highly aggressive bone and soft tissue tumor affecting children and young adults. As other solid tumors, EWS are reliant on a functional vascular network for the delivery of nutrients and oxygen and for the removal of waste. Based on the biological roles of EphA2 in promoting angiogenesis, we explored the functional role of this receptor and its relationship with caveolin-1 (CAV1) in EWS angiogenesis. We demonstrated that lack of CAV1 results in a significant reduction in micro vascular density (MVD) on 3 different in vivo models. In vitro, this phenomenon correlated with inactivation of EphA2 receptor, lack of AKT response and downregulation of bFGF. We also demonstrated that secreted bFGF from EWS cells acted as chemoattractant for endothelial cells. Furthermore, interaction between EphA2 and CAV1 was necessary for the right localization and signaling of the receptor to produce bFGF through AKT and promote migration of endothelial cells. Finally, introduction of a dominant-negative form of EphA2 into EWS cells mostly reproduced the effects occurred by CAV1 silencing, strongly suggesting that the axis EphA2-CAV1 participates in the promotion of endothelial cell migration toward the tumors favoring EWS angiogenesis. | | 23951165
 |
Differential gene expression in eyecup and retina of a mouse model of Stargardt-like macular dystrophy (STGD3).
Kuny, S; Gaillard, F; Sauvé, Y
Investigative ophthalmology & visual science
53
664-75
2011
Zobrazit abstrakt
To investigate differentially expressed genes in eyecup and retina of the ELOVL4 transgenic mouse, a model of Stargardt-like macular dystrophy (STGD3).We examined gene and protein expression in known pathways relevant to retinal degeneration using PCR arrays, Western blotting, and immunohistochemistry. Investigations were performed on ELOVL4 transgenic mice at 9 months, when 50% of rod (but no cone) photoreceptors had degenerated. Age-matched wild-type littermates served as controls.Significant expression level changes were found in only 17 of the 252 genes examined. Nine were upregulated (Fgf2, Fgfr1, Ntf5, Cbln1, Ngfr, Ntrk1, Trp53, Tlr6, and Herpud1), and eight were downregulated (Ccl22, Ccr3, Il18rap, Nf1, Ccl11, Atf6β, Rpn1, and Serp1). Overexpression of FGF2 was detected at 1 month, before rod loss onset, and was maintained at high levels until cone loss (18 months). By 9 months, FGF2 overexpression was seen in photoreceptor cell bodies. Increased glial fibrillary acidic protein (GFAP) expression due to glial cell reactivity followed the same time course. Levels of NGFR/p75NTR remained invariant. Although present in rod outer segments at 1 month, the macrophage chemoattracting chemokine CCL22 became undetectable by 9 months, a likely consequence of progressive rod outer segment truncation.At a mid-degeneration stage, major changes in gene expression in the ELOVL4 transgenic mouse retina included upregulation of Fgf2 and Fgfr1 and downregulation of Ccl22. Modulation of FGF2 occurred very early, concomitant with an increase in GFAP expression. Future studies will address which factors upstream of Fgf2 could provide potential therapeutic targets to slow photoreceptor degeneration in STGD3. | | 22199241
 |
Increased fibroblast telomerase expression precedes myofibroblast α-smooth muscle actin expression in idiopathic pulmonary fibrosis.
Waisberg, DR; Parra, ER; Barbas-Filho, JV; Fernezlian, S; Capelozzi, VL
Clinics (São Paulo, Brazil)
67
1039-46
2011
Zobrazit abstrakt
This study sought to identify the relationship between fibroblast telomerase expression, myofibroblasts, and telomerase-mediated regulatory signals in idiopathic pulmonary fibrosis.Thirty-four surgical lung biopsies, which had been obtained from patients with idiopathic pulmonary fibrosis and histologically classified as usual interstitial pneumonia, were examined. Immunohistochemistry was used to evaluate fibroblast telomerase expression, myofibroblast α-smooth muscle actin expression and the tissue expression of inter leu kin-4, transforming growth factor-β, and basic fibroblast growth factor. The point-counting technique was used to quantify the expression of these markers in unaffected, collapsed, mural fibrosis, and honeycombing areas. The results were correlated to patient survival.Fibroblast telomerase expression and basic fibroblast growth factor tissue expression were higher in collapsed areas, whereas myofibroblast expression and interleukine-4 tissue expression were higher in areas of mural fibrosis. Transforming growth factor-β expression was higher in collapsed, mural fibrosis and honeycombing areas in comparison to unaffected areas. Positive correlations were found between basic fibroblast growth factor tissue expression and fibroblast telomerase expression and between interleukin-4 tissue expression and myofibroblast α-smooth muscle actin expression. Negative correlations were observed between interleukin-4 expression and basic fibroblast growth factor tissue expression in areas of mural fibrosis. Myofibroblast α-smooth muscle actin expression and interleukin-4 tissue expression in areas of mural fibrosis were negatively associated with patient survival.Fibroblast telomerase expression is higher in areas of early remodeling in lung tissues demonstrating typical interstitial pneumonia, whereas myofibroblast α-smooth muscle actin expression predominates in areas of late remodeling. These events seem to be regulated by basic fibroblast growth factor and interleukin-4 tissue expression, respectively. | | 23018301
 |
Time-coordinated prevalence of extracellular HGF, FGF2 and TGF-β3 in crush-injured skeletal muscle.
Mai-Khoi Q DO,Takahiro Suzuki,Borjigin Gerelt,Yusuke Sato,Wataru Mizunoya,Mako Nakamura,Yoshihide Ikeuchi,Judy E Anderson,Ryuichi Tatsumi
Animal science journal = Nihon chikusan Gakkaihō
83
2011
Zobrazit abstrakt
Successful regeneration and remodeling of neuromuscular junctions are critical for restoring functional capacities and properties of skeletal muscle after damage, and axon-guidance molecules may be involved in the signaling that regulates such restoration. Recently, we found that early-differentiated satellite cells up-regulate a secreted neural chemorepellent Sema3A upon in vivo muscle-crush injury. The study also revealed that Sema3A expression is up-regulated in primary satellite-cell cultures in response to hepatocyte growth factor (HGF) and basic fibroblast growth factor (FGF2) and is prevented by transforming growth factor (TGF)-β2, 3. In order to verify the physiological significance of this regulation in vitro, the present study was designed to estimate the time-course of extracellular HGF, FGF2 and TGF-β3 concentrations after crush-injury of Gastrocnemius muscle in the rat lower hind-limb, using a combination of a non-homogenization/non-spin extraction of extracellular wound fluids and enhanced chemiluminescence-Western blotting analyses. Results clearly demonstrated that active HGF and FGF2 are prevalent in 2-8 days post-crush, whereas active TGF-β3 increases after 12 days, providing a better understanding of the time-coordinated levels of HGF, FGF2 and TGF-β3 that drive regulation of Sema3A expression during regenerative intramuscular moto-neuritogenesis. | | 23035711
 |
Preferential accumulation and export of high molecular weight FGF-2 by rat cardiac non-myocytes.
Santiago, JJ; Ma, X; McNaughton, LJ; Nickel, BE; Bestvater, BP; Yu, L; Fandrich, RR; Netticadan, T; Kardami, E
Cardiovascular research
89
139-47
2010
Zobrazit abstrakt
fibroblast growth factor-2 (FGF-2), implicated in paracrine induction of cardiac hypertrophy, is translated as high molecular weight (Hi-FGF-2) and low molecular weight (Lo-FGF-2) isoforms. Paracrine activities are assigned to Lo-FGF-2, whereas Hi-FGF-2 is presumed to have nuclear functions. In this work, we re-examined the latter presumption by asking whether: cardiac non-myocytes (CNMs) accumulate and export Hi-FGF-2 in response to pro-hypertrophic [angiotensin II (Ang II)] stimuli; an unconventional secretory pathway requiring activated caspase-1 affects Hi-FGF2 export; and secreted Hi-FGF-2 is pro-hypertrophic.using neonatal rat heart-derived cultures and immunoblotting, we show that CNMs accumulated over 90% Hi-FGF-2, at levels at least five-fold higher than cardiomyocytes (CMs). Pro-hypertrophic agents (Ang II, endothelin-1, and isoproterenol) up-regulated CNM-associated Hi-FGF-2. The Ang II effect was mediated by Ang II receptor-1 but not Ang II receptor-2 as it was blocked by losartan but not PD123319. CNM-derived Hi-FGF-2 was detected in two extracellular pools: in conditioned medium from Ang II-stimulated CNMs and in association with the cell surface/matrix, eluted with a gentle 2 M NaCl wash of the cell monolayer. Conditioned medium from Ang II-treated CNMs increased neonatal CM size, an effect prevented by anti-FGF-2-neutralizing antibodies. The caspase-1 inhibitor YVAD prevented the Ang II-induced release of Hi-FGF-2 to both extracellular pools.CNMs are major producers of Hi-FGF-2, up-regulated by hypertrophic stimuli and exported to the extracellular environment by a mechanism requiring caspase-1 activity, suggesting a link to the innate immune response. Hi-FGF-2 is likely to promote paracrine induction of myocyte hypertrophy in vivo. | Western Blotting | 20696821
 |
Renal collecting system growth and function depend upon embryonic γ1 laminin expression.
Yang, DH; McKee, KK; Chen, ZL; Mernaugh, G; Strickland, S; Zent, R; Yurchenco, PD
Development (Cambridge, England)
138
4535-44
2010
Zobrazit abstrakt
In order to understand the functions of laminins in the renal collecting system, the Lamc1 gene was inactivated in the developing mouse ureteric bud (UB). Embryos bearing null alleles exhibited laminin deficiency prior to mesenchymal tubular induction and either failed to develop a UB with involution of the mesenchyme, or developed small kidneys with decreased proliferation and branching, delayed renal vesicle formation and postnatal emergence of a water transport deficit. Embryonic day 12.5 kidneys revealed an almost complete absence of basement membrane proteins and reduced levels of α6 integrin and FGF2. mRNA levels for fibroblast growth factor 2 (FGF2) and mediators of the GDNF/RET and WNT11 signaling pathway were also decreased. Furthermore, collecting duct cells derived from laminin-deficient kidneys and grown in collagen gels were found to proliferate and branch slowly. The laminin-deficient cells exhibited decreased activation of growth factor- and integrin-dependent pathways, whereas heparin lyase-treated and β1 integrin-null cells exhibited more selective decreases. Collectively, these data support a requirement of γ1 laminins for assembly of the collecting duct system basement membrane, in which immobilized ligands act as solid-phase agonists to promote branching morphogenesis, growth and water transport functions. | | 21903675
 |
Matrix metalloproteinases and angiogenic factors: predictors of survival after radical prostatectomy for clinically organ-confined prostate cancer?
Boxler, S; Djonov, V; Kessler, TM; Hlushchuk, R; Bachmann, LM; Held, U; Markwalder, R; Thalmann, GN
The American journal of pathology
177
2216-24
2009
Zobrazit abstrakt
The aim of the present study was to investigate whether biomarkers improve the prediction of recurrence-free, disease-specific, and overall survival in patients with clinically localized prostate cancer. A tissue microarray was constructed from prostate specimens of 278 patients who underwent open radical retropubic prostatectomy for clinically localized prostate cancer. For immunohistochemical studies, antibodies were used against matrix metalloproteinase (MMP)-2, MMP-3, MMP-7, MMP-9, MMP-13, and MMP-19, as well as against vascular endothelial growth factor, hypoxia-induced factor 1α, basic fibroblast growth factor, and cluster of differentiation 31. Univariate and multivariable analyses were performed to evaluate the potential predictors of overall, disease-specific, and recurrence-free survival. In univariate analysis of patients with clinically organ-confined prostate cancer, only higher expression levels of MMP-9 (hazard ratio [0.6], 95% CI 0.45-0.8) had a protective effect in terms of overall survival. This positive effect of high MMP-9 expression was also observed for recurrence-free (HR 0.88, 95% CI 0.78-0.99) and disease-specific survival (HR 0.5, 95% CI 0.36-0.73). In multivariable analysis, none of these potential markers was found to be an independent prognostic factor of survival. Of all MMPs and angiogenic factors tested, MMP-9 expression has the potential as a prognostic marker in patients undergoing radical prostatectomy for clinically organ-confined cases of prostate cancer. | Immunohistochemistry | 20889560
 |
Membrane vesicles containing matrix metalloproteinase-9 and fibroblast growth factor-2 are released into the extracellular space from mouse mesoangioblast stem cells.
Candela, Maria Elena, et al.
J. Cell. Physiol., 224: 144-51 (2010)
2009
Zobrazit abstrakt
Certain proteins, including fibroblast growth factor-2 (FGF-2) and matrix metalloproteinase-9 (MMP-9), have proved very effective in increasing the efficacy of mesoangioblast stem cell therapy in repairing damaged tissue. We provide the first evidence that mouse mesoangioblast stem cells release FGF-2 and MMP-9 in their active form through the production of membrane vesicles. These vesicles are produced and turned over continuously, but are stable for some time in the extracellular milieu. Mesoangioblasts shed membrane vesicles even under oxygen tensions that are lower than those typically used for cell culture and more like those of mouse tissues. These findings suggest that mesoangioblasts may themselves secrete paracrine signals and factors that make damaged tissues more amenable to cell therapy through the release of membrane vesicles. | | 20232295
 |
beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity.
Xi Zhang,Glenda Mernaugh,Dong-Hua Yang,Leslie Gewin,Manakan B Srichai,Raymond C Harris,Juan M Iturregui,Raoul D Nelson,Donald E Kohan,Dale Abrahamson,Reinhard Fässler,Peter Yurchenco,Ambra Pozzi,Roy Zent
Development (Cambridge, England)
136
2009
Zobrazit abstrakt
The kidney collecting system develops from branching morphogenesis of the ureteric bud (UB). This process requires signaling by growth factors such as glial cell line derived neurotrophic factor (GDNF) and fibroblast growth factors (FGFs) as well as cell extracellular matrix interactions mediated by integrins. The importance of integrin signaling in UB development was investigated by deleting integrin beta1 at initiation (E10.5) and late (E18.5) stages of development. Deletion at E10.5 resulted in a severe branching morphogenesis phenotype. Deletion at E18.5 did not alter renal development but predisposed the collecting system to severe injury following ureteric obstruction. beta1 integrin was required for renal tubular epithelial cells to mediate GDNF- and FGF-dependent signaling despite normal receptor localization and activation in vitro. Aberrations in the same signaling molecules were present in the beta1-null UBs in vivo. Thus beta1 integrins can regulate organ branching morphogenesis during development by mediating growth-factor-dependent signaling in addition to their well-defined role as adhesion receptors. Celý text článku | | 19710172
 |