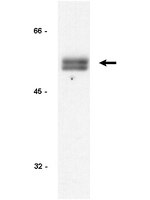
Krüppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts.
El-Karim, EA; Hagos, EG; Ghaleb, AM; Yu, B; Yang, VW
Molecular cancer
12
89
2013
Zobrazit abstrakt
Krüppel-like factor 4 (KLF4) is a member of the KLF family of transcription factors and regulates proliferation, differentiation, apoptosis and somatic cell reprogramming. Evidence also suggests that KLF4 is a tumor suppressor in certain cancers including colorectal cancer. We previously showed that KLF4 inhibits cell cycle progression following DNA damage and that mouse embryonic fibroblasts (MEFs) null for Klf4 are genetically unstable, as evidenced by increased rates of cell proliferation, and the presence of DNA double strand breaks (DSBs), centrosome amplification, chromosome aberrations and aneuploidy.To determine whether re-expression of Klf4 corrects the observed genetic instability in MEFs null for Klf4 (Klf4(-/-)), we transfected Klf4(-/-)MEFs with Klf4-expressing plasmids and compared the results to wild type (Klf4(+/+)) and untransfected or mock-transfected Klf4(-/-)MEFs.We show that overexpression of Klf4 in Klf4(-/-)MEFs reduced cell proliferation rates and the proportion of cells with DSBs, abnormal centrosome numbers, aneuploidy and micronuclei. In addition, Klf4-transfected Klf4(-/-)MEFs exhibited a more robust DNA damage repair response as demonstrated by the greater rate in disappearance of γ-H2AX and 53BP1 foci following γ-irradiation.Taken together these findings provide evidence that KLF4 plays a crucial role in the maintenance of genetic stability by modulating the DNA damage response and repair processes. | Immunofluorescence | 23919723
 |
Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells.
Maimets, T; Neganova, I; Armstrong, L; Lako, M
Oncogene
27
5277-87
2008
Zobrazit abstrakt
p53 Is an important regulator of normal cell response to stress and frequently mutated in human tumours. Here, we studied the effects of activation of p53 and its target gene p21 in human embryonic stem cells. We show that activation of p53 with small-molecule activator nutlin leads to rapid differentiation of stem cells evidenced by changes in cell morphology and adhesion, expression of cell-specific markers for primitive endoderm and trophectoderm lineages and loss of pluripotency markers. p21 is quickly and dose-dependently activated by nutlin. It can also be activated independently from p53 by sodium butyrate, which leads to the differentiation events very similar to the ones induced by p53. During differentiation, the activating phosphorylation site of CDK2 Thr-160 becomes dephosphorylated and cyclins A and E become degraded. The target for CDK2 kinase in p53 molecule, Ser-315, also becomes dephosphorylated. We conclude that the main mechanism responsible for differentiation of human stem cells by p53 is abolition of S-phase entry and subsequent stop of cell cycle in G0/G1 phase accompanied by p21 activation. | Western Blotting | 18521083
 |
Differential gene signatures in rat mammary tumors induced by DMBA and those induced by fractionated gamma radiation.
Hae-June Lee, Yoon-Jin Lee, Chang-Mo Kang, Sangwoo Bae, Dooil Jeoung, Ja-June Jang, Seung-Sook Lee, Chul-Koo Cho, Yun-Sil Lee
Radiation research
170
579-90
2008
Zobrazit abstrakt
The aim of this work was to identify specific genes involved in rat mammary tumors induced by dimethylbenz(a)anthracene (DMBA) or radiation. More TUNEL- and PCNA-positive cells were present in mammary tumors induced by radiation than in tumors induced by DMBA, whereas DNA damage responses like p53 accumulation and histone H2AX phosphorylation were higher in DMBA-induced tumors, even though the pathology was similar in both types of tumors. cDNA microarray and real-time RT-PCR analysis of radiation- or DMBA-induced tumor tissues, revealed that stanniocalcin 2 (Stc2), interferon regulatory factor 1 (Irf1), interleukin 18 binding protein (Il18bp), and chloride channel calcium activated 3 (Clca3) were expressed in both, and that arachidonate 5-lipoxygenase activating protein 1 (Alox5ap) and cathepsin S (Ctss) were expressed only in radiation-induced tumors. No DMBA-specific gene signatures were found. Soft agar growth assays were carried out to identify the carcinogenic features of these specific genes. Cells stably transfected with Alox5ap, Ctss, Stc2, Irf1, Il18bp and Clca3 showed morphological changes compared to controls. These findings indicate different gene alterations in carcinogen- or radiation-induced mammary tumors with similar pathological stages. | | 18959458
 |
IKK alpha regulates estrogen-induced cell cycle progression by modulating E2F1 expression.
Tu, Z; Prajapati, S; Park, KJ; Kelly, NJ; Yamamoto, Y; Gaynor, RB
The Journal of biological chemistry
281
6699-706
2005
Zobrazit abstrakt
The IkappaB kinase (IKK) complex consists of the catalytic subunits IKKalpha and IKKbeta and a regulatory subunit, IKKgamma/NEMO. Even though IKKalpha and IKKbeta share significant sequence similarity, they have distinct biological roles. It has been demonstrated that IKKs are involved in regulating the proliferation of both normal and tumor cells, although the mechanisms by which they function in this process remain to be better defined. In this study, we demonstrate that IKKalpha, but not IKKbeta, is important for estrogen-induced cell cycle progression by regulating the transcription of the E2F1 gene as well as other E2F1-responsive genes, including thymidine kinase 1, proliferating cell nuclear antigen, cyclin E, and cdc25A. The role of IKKalpha in regulating E2F1 was not the result of reduced levels of cyclin D1, as overexpression of this gene could not overcome the effects of IKKalpha knock-down. Furthermore, estrogen treatment increased the association of endogenous IKKalpha and E2F1, and this interaction occurred on promoters bound by E2F1. IKKalpha also potentiated the ability of p300/CBP-associated factor to acetylate E2F1. Taken together, these data suggest a novel mechanism by which IKKalpha can influence estrogen-mediated cell cycle progression through its regulation of E2F1. | Fluorescence Activated Cell Sorting (FACS) | 16407216
 |
Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2.
Lents, Nathan H, et al.
J. Biol. Chem., 277: 47469-75 (2002)
2002
Zobrazit abstrakt
The activity of cyclin-dependent kinase 2 is required for G(1)-S-phase progression of the eukaryotic cell cycle. In this study, we examine the activation of CDK2-cyclin E by constructing a CDK2 that is constitutively targeted to the nucleus. Activation of CDK2 requires the removal of two inhibitory phosphates (Thr-14 and Tyr-15) and the addition of one activating phosphate (Thr-160) by a nuclear localized CDK-activating kinase, which is thought to be constitutively active. Surprisingly, nuclear localized CDK2-NLS and CDK2-NLS(A14,F15), which lacks the inhibitory phosphorylation sites, require serum to become active, despite complexing with expressed cyclin E. We show that inhibition of mitogen-mediated ERK activation by treatment with U0126, a selective MEK inhibitor, or expression of dominant-negative ERK markedly reduces the phosphorylation of Thr-160 and enzymatic activity of both CDK2-NLS constructs. Consistent with a role for ERK in Thr-160 phosphorylation, expression of constitutively active Raf-1 induces Thr-160 phosphorylation of CDK2-NLS in serum-arrested cells, an effect that is blocked by treatment with U0126. Taken together, these data show a new role for ERK in G1 cell cycle progression: In addition to its role in stimulating cyclin D1 expression and nuclear translocation of CDK2, ERK regulates Thr-160 phosphorylation of CDK2-cyclin E. | Immunoprecipitation | 12359725
 |
Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase.
Keenan, S M, et al.
J. Biol. Chem., 276: 22404-9 (2001)
2001
Zobrazit abstrakt
Activation of cyclin-dependent kinase 2 (CDK2)-cyclin E in the late G(1) phase of the cell cycle is important for transit into S phase. In Chinese hamster embryonic fibroblasts (IIC9) phosphatidylinositol 3-kinase and ERK regulate alpha-thrombin-induced G(1) transit by their effects on cyclin D1 protein accumulation (Phillips-Mason, P. J., Raben, D. M., and Baldassare, J. J. (2000) J. Biol. Chem. 275, 18046-18053). Here, we show that ERK also affects CDK2-cyclin E activation by regulating the subcellular localization of CDK2. Ectopic expression of cyclin E rescues the inhibition of alpha-thrombin-induced activation of CDK2-cyclin E and transit into S phase brought about by treatment of IIC9 cells with LY29004, a selective inhibitor of mitogen stimulation of phosphatidylinositol 3-kinase activity. However, cyclin E expression is ineffectual in rescuing these effects when ERK activation is blocked by treatment with PD98059, a selective inhibitor of MEK activation of ERK. Investigation into the mechanistic reasons for this difference found the following. 1) Although treatment with LY29004 inhibits alpha-thrombin-stimulated nuclear localization, ectopic expression of cyclin E rescues CDK2 translocation. 2) In contrast to treatment with LY29004, ectopic expression of cyclin E fails to restore alpha-thrombin-stimulated nuclear CDK2 translocation in IIC9 cells treated with PD98059. 3) CDK2-cyclin E complexes are not affected by treatment with either inhibitor. These data indicate that, in addition to its effects on cyclin D1 expression, ERK activity is an important controller of the translocation of CDK2 into the nucleus where it is activated. | Immunoblotting (Western) | 11304535
 |