Fibrochondrogenic potential of synoviocytes from osteoarthritic and normal joints cultured as tensioned bioscaffolds for meniscal tissue engineering in dogs.
Warnock, JJ; Bobe, G; Duesterdieck-Zellmer, KF
PeerJ
2
e581
2014
Zobrazit abstrakt
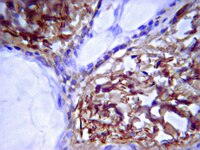
Meniscal tears are a common cause of stifle lameness in dogs. Use of autologous synoviocytes from the affected stifle is an attractive cell source for tissue engineering replacement fibrocartilage. However, the diseased state of these cells may impede in vitro fibrocartilage formation. Synoviocytes from 12 osteoarthritic ("oaTSB") and 6 normal joints ("nTSB") were cultured as tensioned bioscaffolds and compared for their ability to synthesize fibrocartilage sheets. Gene expression of collagens type I and II were higher and expression of interleukin-6 was lower in oaTSB versus nTSB. Compared with nTSB, oaTSB had more glycosaminoglycan and alpha smooth muscle staining and less collagen I and II staining on histologic analysis, whereas collagen and glycosaminoglycan quantities were similar. In conclusion, osteoarthritic joint-origin synoviocytes can produce extracellular matrix components of meniscal fibrocartilage at similar levels to normal joint-origin synoviocytes, which makes them a potential cell source for canine meniscal tissue engineering. | | 25289180
 |
In vitro synthesis of tensioned synoviocyte bioscaffolds for meniscal fibrocartilage tissue engineering.
Warnock, JJ; Baker, L; Ballard, GA; Ott, J
BMC veterinary research
9
242
2013
Zobrazit abstrakt
Meniscal injury is a common cause of lameness in the dog. Tissue engineered bioscaffolds may be a treatment option for meniscal incompetency, and ideally would possess meniscus- like extracellular matrix (ECM) and withstand meniscal tensile hoop strains. Synovium may be a useful cell source for meniscal tissue engineering because of its natural role in meniscal deficiency and its in vitro chondrogenic potential. The objective of this study is to compare meniscal -like extracellular matrix content of hyperconfluent synoviocyte cell sheets ("HCS") and hyperconfluent synoviocyte sheets which have been tensioned over wire hoops (tensioned synoviocyte bioscaffolds, "TSB") and cultured for 1 month.Long term culture with tension resulted in higher GAG concentration, higher chondrogenic index, higher collagen concentration, and type II collagen immunoreactivity in TSB versus HCS. Both HCS and TSB were immunoreactive for type I collagen, however, HCS had mild, patchy intracellular immunoreactivity while TSB had diffuse moderate immunoreactivity over the entire bisocaffold. The tissue architecture was markedly different between TSB and HCS, with TSB containing collagen organized in bands and sheets. Both HCS and TSB expressed alpha smooth muscle actin and displayed active contractile behavior. Double stranded DNA content was not different between TSB and HCS, while cell viability decreased in TSB.Long term culture of synoviocytes with tension improved meniscal- like extra cellular matrix components, specifically, the total collagen content, including type I and II collagen, and increased GAG content relative to HCS. Future research is warranted to investigate the potential of TSB for meniscal tissue engineering. | Immunohistochemistry | 24299420
 |
Synthetic triterpenoids, CDDO-Imidazolide and CDDO-Ethyl amide, induce chondrogenesis.
N Suh,S Paul,H J Lee,T Yoon,N Shah,A I Son,A H Reddi,D Medici,M B Sporn
Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society
20
2011
Zobrazit abstrakt
Novel methods for inducing chondrogenesis are critical for cartilage tissue engineering and regeneration. Here we show that the synthetic oleanane triterpenoids, CDDO-Imidazolide (CDDO-Im) and CDDO-Ethyl amide (CDDO-EA), at concentrations as low as 200 nM, induce chondrogenesis in organ cultures of newborn mouse calvaria. The cartilage phenotype was measured histologically with metachromatic toluidine blue staining for proteoglycans and by immunohistochemical staining for type II collagen. Furthermore, real-time polymerase chain reaction (PCR) analysis using mRNA from calvaria after 7-day treatment with CDDO-Im and CDDO-EA showed up-regulation of the chondrocyte markers SOX9 and type II collagen (alpha1). In addition, TGF-β; BMPs 2 and 4; Smads 3, 4, 6, and 7; and TIMPs-1 and -2 were increased. In contrast, MMP-9 was strongly down-regulated. Treatment of human bone marrow-derived mesenchymal stem cells with CDDO-Im and CDDO-EA (100 nM) induced expression of SOX9, collagen IIα1, and aggrecan, as well as BMP-2 and phospho-Smad5, confirming that the above triterpenoids induce chondrogenic differentiation. This is the first report of the use of these drugs for induction of chondrogenesis. | | 22343171
 |
Hypoxic expansion promotes the chondrogenic potential of articular chondrocytes.
Rainer J Egli,Johannes D Bastian,Reinhold Ganz,Willy Hofstetter,Michael Leunig
Journal of orthopaedic research : official publication of the Orthopaedic Research Society
26
2008
Zobrazit abstrakt
For cell-based cartilage repair strategies, an ex vivo expansion phase is required to obtain sufficient numbers of cells needed for therapy. Although recent reports demonstrated the central role of oxygen for the function and differentiation of chondrocytes, a beneficial effect of low oxygen concentrations during the expansion of the cells to further improve their chondrogenic capacity has not been investigated.Therefore, freshly harvested bovine articular chondrocytes were grown in two-dimensional monolayer cultures at 1.5% and 21% O2 and redifferentiation was subsequently induced in three-dimensional micromass cultures at 1.5%, 5%, and 21% O2. Cells expanded at 1.5% O2 were characterized by low citrate synthase (aerobic energy metabolism)--and high LDH (anaerobic energy metabolism-activities,suggesting an anaerobic energy metabolism. Collagen type II mRNA was twofold higher in cells expanded at 1.5% as compared to expansion at 21% O2. Micromass cultures grown at 21% O2 showed up to a twofold increase in the tissue content of glycosaminoglycans when formed with cells expanded at 1.5% instead of 21% O2. However, no differences in the levels of transcripts and in the staining for collagen type II protein were observed in these micromass cultures. Hypoxia (1.5% and 5% O2) applied during micromass cultures gave rise to tissues with low contents of glycosaminoglycans only. In vivo, the chondrocytes are adapted to a hypoxic environment. Taking this into account, by applying 1.5% O2 in the expansion phase in the course of cell-based cartilage repair strategies, may result in a repair tissue with higher quality by increasing the content of glycosaminoglycans. | | 18302236
 |