112252 Sigma-AldrichAcidic Mammalian Chitinase Inhibitor, Bisdionin F - CAS 917877-86-2 - Calbiochem
The Acidic Mammalian Chitinase Inhibitor, Bisdionin F controls the biological activity of Acidic Mammalian Chitinase.
More>> The Acidic Mammalian Chitinase Inhibitor, Bisdionin F controls the biological activity of Acidic Mammalian Chitinase. Less<<Synonyms: AMCase Inhibitor, 3,7-dimethyl-1-(3-(3-methyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-1-yl)propyl)-1H-purine-2,6(3H,7H)-dione
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
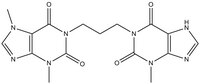
| 917877-86-2 | C₁₆H₁₈N₈O₄ |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 112252-5MG | Skleněná láhev | 5 mg |
| References | |
|---|---|
| References | Sutherland, T.E., et al. 2011. Chem. Biol. 18, 569. |
| Product Information | |
|---|---|
| CAS number | 917877-86-2 |
| Form | Off-white powder |
| Hill Formula | C₁₆H₁₈N₈O₄ |
| Chemical formula | C₁₆H₁₈N₈O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 112252-5MG | 04055977208030 |
Documentation
Acidic Mammalian Chitinase Inhibitor, Bisdionin F - CAS 917877-86-2 - Calbiochem MSDS
| Title |
|---|
Acidic Mammalian Chitinase Inhibitor, Bisdionin F - CAS 917877-86-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 112252 |
References
| Přehled odkazů |
|---|
| Sutherland, T.E., et al. 2011. Chem. Biol. 18, 569. |