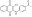530197 Sigma-AldrichCD45 Inhibitor VI - CAS 130089-98-4 - Calbiochem
A cell-permeable, potent, irreversible, non-substrate competitive inhibitor of CD45/PTPRC tyrosine phosphatase activity (IC₅₀ = 290 nM).
More>> A cell-permeable, potent, irreversible, non-substrate competitive inhibitor of CD45/PTPRC tyrosine phosphatase activity (IC₅₀ = 290 nM). Less<<Synonyms: 2-(4-Acetylanilino)-3-chloronaphthoquinone, Compound 211, Protein Tyrosine Phosphatase CD45 Inhibitor VI, PTP Inhibitor XXXII
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 130089-98-4 | C₁₈H₁₂ClNO₃ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.30197.0001CN |
|
Glass bottle | 10 mg |
|
— |
| References | |
|---|---|
| References | Perron, M., et al. 2014. Mol. Pharmacol. 85, 553. |
| Product Information | |
|---|---|
| CAS number | 130089-98-4 |
| Form | Orange powder |
| Hill Formula | C₁₈H₁₂ClNO₃ |
| Chemical formula | C₁₈H₁₂ClNO₃ |
| Reversible | N |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | CD45 |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.30197.0001CN | 04055977261301 |
Documentation
CD45 Inhibitor VI - CAS 130089-98-4 - Calbiochem SDS
| Title |
|---|
CD45 Inhibitor VI - CAS 130089-98-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 530197 |
References
| Reference overview |
|---|
| Perron, M., et al. 2014. Mol. Pharmacol. 85, 553. |