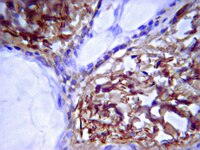
Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: superiority of meniscus as a cell source for meniscus repair.
Ding, Z; Huang, H
BMC musculoskeletal disorders
16
65
2015
Show Abstract
The restoration of damaged meniscus has always been a challenge due to its limited healing capacity. Recently, bone marrow-derived mesenchymal stem cells (BMSCs) provide a promising alternative to repair meniscal defects. However, BMSCs are not ideal chondroprogenitor cells for meniscus repair because they have a high propensity for cartilage hypertrophy and bone formation. Our hypothesis is that mesenchymal stem cells (MSCs) reside in meniscus maintain specific traits distinct from others which may be more conducive to meniscus regeneration.MSCs were isolated from bone marrow and menisci of the rabbits. The similarities and differences between BMSCs and MMSCs were investigated in vitro by a cell culture model, ex vivo by a rabbit meniscus defect model and in vivo by a nude rat implantation model using histochemistry, immunocytochemistry, qRT-PCR and western blotting.Our data showed that two types of MSCs have universal stem cell characteristics including clonogenicity, multi-potency and self-renewal capacity. They both express stem cell markers including SSEA-4, Nanog, nucleostemin, strol-1, CD44 and CD90. However, MMSCs differed from BMSCs. MMSC colonies were much smaller and grew more slowly than BMSC colonies. Moreover, fewer MMSCs expressed CD34 than BMSCs. Finally, MMSCs always appeared a pronounced tendency to chondrogenic differentiation while BMSCs exhibited significantly greater osteogenic potential, whatever in vitro and in vivo.This study shows the similarities and differences between MMSCs and BMSCs for the first time. MMSCs are a promising source of mesenchymal stem cells in repairing meniscus defect. | 25887689
 |
Human tendon stem cells better maintain their stemness in hypoxic culture conditions.
Zhang, J; Wang, JH
PloS one
8
e61424
2013
Show Abstract
Tissues and organs in vivo are under a hypoxic condition; that is, the oxygen tension is typically much lower than in ambient air. However, the effects of such a hypoxic condition on tendon stem cells, a recently identified tendon cell, remain incompletely defined. In cell culture experiments, we subjected human tendon stem cells (hTSCs) to a hypoxic condition with 5% O2, while subjecting control cells to a normaxic condition with 20% O2. We found that hTSCs at 5% O2 had significantly greater cell proliferation than those at 20% O2. Moreover, the expression of two stem cell marker genes, Nanog and Oct-4, was upregulated in the cells cultured in 5% O2. Finally, in cultures under 5% O2, more hTSCs expressed the stem cell markers nucleostemin, Oct-4, Nanog and SSEA-4. In an in vivo experiment, we found that when both cell groups were implanted with tendon-derived matrix, more tendon-like structures formed in the 5% O2 treated hTSCs than in 20% O2 treated hTSCs. Additionally, when both cell groups were implanted with Matrigel, the 5% O2 treated hTSCs showed more extensive formation of fatty, cartilage-like and bone-like tissues than the 20% O2 treated cells. Together, the findings of this study show that oxygen tension is a niche factor that regulates the stemness of hTSCs, and that less oxygen is better for maintaining hTSCs in culture and expanding them for cell therapy of tendon injuries. | 23613849
 |
Characterization of the urethral plate and the underlying tissue defined by expression of collagen subtypes and microarchitecture in hypospadias.
Hayashi Y, Mizuno K, Kojima Y, Moritoki Y, Nishio H, Kato T, Kurokawa S, Kamisawa H, Kohri K
International journal of urology : official journal of the Japanese Urological Association
2011
Show Abstract
Objectives: In hypospadia patients, the urethral plate and the underlying tissue were previously thought to be the main cause of penile curvature and, because of this, they used to be excised to correct the curvature. Currently, they are preserved as they are not thought to cause penile curvature anymore. The aim of the present histology study was to elucidate the characteristic structure of the tissue beneath the urethral plate. Methods: The experimental group consisted of 27 hypospadiac patients with moderately severe penile curvature, who underwent one-stage urethroplasty after dividing the urethral plate. Excised tissues were observed under light microscopy and transmission electron microscopy (TEM). Furthermore, the presence of collagen subtypes I, III and IV was examined with immunohistochemical staining and western blotting. Results: Light microscopy showed the existence of many massed and intertwined collagen fibers and vessels that resembled those of the cavernous sinus. TEM showed the existence of many collagen fibers, capillary vessels and other structures. Immunohistochemical staining showed collagen subtype I in the interfascicular space and collagen fibers were densely stained. Collagen subtype IV was found in the basement membrane of vessels, but collagen subtype III was not detected. The same results were obtained by western blotting. Conclusions: The tissue beneath the urethral plate was considered to originate from the corpus spongiosum penis. The distribution of collagen subtypes suggests that the presence of the tissue might affect ventral penile curvature. Long-term follow up is required after one-stage hypospadias repair with preservation of the urethral plate and the underlying tissue.© 2011 The Japanese Urological Association. | 21332824
 |
Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years.
Alberto Ferruzzi, Roberto Buda, Cesare Faldini, Francesca Vannini, Francesco Di Caprio, Deianira Luciani, Sandro Giannini
The Journal of bone and joint surgery. American volume
90 Suppl 4
90-101
2008
| 18984722
 |
Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence.
Zoumi, A; Yeh, A; Tromberg, BJ
Proceedings of the National Academy of Sciences of the United States of America
99
11014-9
2002
Show Abstract
Multiphoton microscopy relies on nonlinear light-matter interactions to provide contrast and optical sectioning capability for high-resolution imaging. Most multiphoton microscopy studies in biological systems have relied on two-photon excited fluorescence (TPEF) to produce images. With increasing applications of multiphoton microscopy to thick-tissue "intravital" imaging, second-harmonic generation (SHG) from structural proteins has emerged as a potentially important new contrast mechanism. However, SHG is typically detected in transmission mode, thus limiting TPEF/SHG coregistration and its practical utility for in vivo thick-tissue applications. In this study, we use a broad range of excitation wavelengths (730-880 nm) to demonstrate that TPEF/SHG coregistration can easily be achieved in unstained tissues by using a simple backscattering geometry. The combined TPEF/SHG technique was applied to imaging a three-dimensional organotypic tissue model (RAFT). The structural and molecular origin of the image-forming signal from the various tissue constituents was determined by simultaneous spectroscopic measurements and confirming immunofluorescence staining. Our results show that at shorter excitation wavelengths (less than 800 nm), the signal emitted from the extracellular matrix (ECM) is a combination of SHG and TPEF from collagen, whereas at longer excitation wavelengths the ECM signal is exclusively due to SHG. Endogenous cellular signals are consistent with TPEF spectra of cofactors NAD(P)H and FAD at all excitation wavelengths. The reflected SHG intensity follows a quadratic dependence on the excitation power, decays exponentially with depth, and exhibits a spectral dependence in accordance with previous theoretical studies. The use of SHG and TPEF in combination provides complementary information that allows noninvasive, spatially localized in vivo characterization of cell-ECM interactions in unstained thick tissues. | 12177437
 |
Lipoprotein lipase in the arterial wall: linking LDL to the arterial extracellular matrix and much more.
Markku O Pentikäinen, Riina Oksjoki, Katariina Oörni, Petri T Kovanen
Arteriosclerosis, thrombosis, and vascular biology
22
211-7
2002
Show Abstract
For low density lipoprotein (LDL) particles to be atherogenic, increasing evidence indicates that their residence time in the arterial intima must be sufficient to allow their modification into forms capable of triggering extracellular and intracellular lipid accumulation. Recent reports have confirmed the longstanding hypothesis that the major determinant(s) of initial LDL retention in the preatherosclerotic arterial intima is the proteoglycans. However, once the initial atherosclerotic lesions have formed, a shift to retention facilitated by macrophage-derived lipoprotein lipase (LPL) appears, leading to the progression of the lesions. Here, we review recent findings on the mechanisms enabling LPL to promote LDL retention and extracellular lipid accumulation in the arterial intima, and we describe the structures in the extracellular matrix that are held to be important in this process. Finally, the potentially harmful consequences of LDL linking by LPL and of other LPL actions in the arterial intima are briefly reviewed. | 11834518
 |
Lipoprotein lipase (LPL) strongly links native and oxidized low density lipoprotein particles to decorin-coated collagen. Roles for both dimeric and monomeric forms of LPL.
M O Pentikäinen, K Oörni, P T Kovanen
The Journal of biological chemistry
275
5694-701
2000
Show Abstract
Low density lipoprotein (LDL) and oxidized LDL are associated with collagen in the arterial intima, where the collagen is coated by the small proteoglycan decorin. When incubated in physiological ionic conditions, decorin-coated collagen bound only small amounts of native and oxidized LDL, the interaction being weak. When decorin-coated collagen was first allowed to bind lipoprotein lipase (LPL), binding of native and oxidized LDL increased dramatically (23- and 7-fold, respectively). This increase depended on strong interactions between LPL that was bound to the glycosaminoglycan chains of the collagen-bound decorin and native and oxidized LDL (kDa 12 and 5.9 nM, respectively). To distinguish between binding to monomeric (inactive) and dimeric (catalytically active) forms of LPL, affinity chromatography on heparin columns was conducted, which showed that native LDL bound to the monomeric LPL, whereas oxidized LDL, irrespective of the type of modification (Cu(2+), 2, 2'-azobis(2-amidinopropane)hydrochloride, hypochlorite, or soybean 15-lipoxygenase), bound preferably to dimeric LPL. However, catalytic activity of LPL was not required for binding to oxidized LDL. Finally, immunohistochemistry of atherosclerotic lesions of human coronary arteries revealed specific areas in which LDL, LPL, decorin, and collagen type I were present. The results suggest that LPL can retain LDL in atherosclerotic lesions along decorin-coated collagen fibers. | 10681554
 |
Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes.
J Chesney, C Metz, A B Stavitsky, M Bacher, R Bucala
Journal of immunology (Baltimore, Md. : 1950)
160
419-25
1998
Show Abstract
We recently described a novel population of blood-borne cells, termed fibrocytes, that display a distinct cell surface phenotype (collagen+/CD13+/CD34+/CD45+), rapidly enter sites of tissue injury, and contribute to scar formation. To further characterize the role of these cells in vivo, we examined the expression of type I collagen and cytokine mRNAs by cells isolated from wound chambers implanted into mice. Five days after chamber implantation, CD34+ fibrocytes but not CD14+ monocytes or CD90+ T cells expressed mRNA for type I collagen. Fibrocytes purified from wound chambers also were found to express mRNA for IL-1beta, IL-10, TNF-alpha, JE/MCP, MIP-1alpha, MIP-1beta, MIP-2, PDGF-A, TGF-beta1, and M-CSF. The addition of IL-1beta (1-100 ng/ml), a critical mediator in wound healing, to fibrocytes isolated from human peripheral blood induced the secretion of chemokines (MIP-1alpha, MIP-1beta, MCP-1, IL-8, and GRO alpha), hemopoietic growth factors (IL-6, IL-10, and macrophage-CSF), and the fibrogenic cytokine TNF-alpha. By contrast, IL-1beta decreased the constitutive secretion of type I collagen as measured by ELISA. Additional evidence for a role for fibrocytes in collagen production in vivo was obtained in studies of livers obtained from Schistosoma japonicum-infected mice. Mouse fibrocytes localized to areas of granuloma formation and connective matrix deposition. We conclude that fibrocytes are an important source of cytokines and type I collagen during both the inflammatory and the repair phase of the wound healing response. Furthermore, IL-1beta may act on fibrocytes to effect a phenotypic transition between a repair/remodeling and a proinflammatory mode. | 9551999
 |
Immunohistochemical characterization of intact stromal layers in long-term cultures of human bone marrow.
Wilkins, B S and Jones, D B
Br. J. Haematol., 90: 757-66 (1995)
1995
Show Abstract
We have performed an immunohistochemical study of intact adherent layers of human long-term bone marrow cultures (hLTBMC) in order to characterize the cell types present. Our panel of antibodies was selected to demonstrate various mesenchymal and haemopoietically derived cell types and to assess the presence of molecules of potential importance as adhesive ligands between haemopoietic cells and stroma. Subpopulations of fibroblasts and macrophages were identified which differed in immunophenotype. We were able to demonstrate modulation of fibroblast and extra-cellular matrix immunophenotypes between 2 and 12 weeks in culture. Stromal cells and matrix expressed a wide variety of antigens of potential importance in haemopoietic cell adhesion, but no ICAM-1, 2 or 3 could be demonstrated to correspond to the strong LFA-1 expression seen in haemopoietic precursor cells. No localization of antigen expression by stromal elements was found to account for the formation of haemopoietic foci at particular sites. However, granulocyte-predominant foci preferentially occupied the interstices and margins of structures which appeared to be vascular arrays. | 7669654
 |
Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes.
B A Roecklein, B Torok-Storb
Blood
85
997-1005
1995
Show Abstract
A replication-defective recombinant retrovirus containing the human papilloma virus E6/E7 genes (LXSN-16 E6E7) was used to immortalize stromal cells from human marrow. The E6/E7 gene products interfere with the function of tumor-suppressor proteins p53 and Rb, respectively, thereby preventing cell cycle arrest without causing significant transformation. Twenty-seven immortalized clones designated HS-1 to HS-27 were isolated, four of which are characterized in this report. Two cell lines, HS-5 and HS-21, appear to be fibroblastoid and secrete significant levels of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage-CSF (GM-CSF), macrophage-CSF (M-CSF), Kit ligand (KL), macrophage-inhibitory protein-1 alpha, interleukin-6 (IL-6), IL-8, and IL-11. However, only HS-5 supports proliferation of hematopoietic progenitor cells when cocultured in serum-deprived media with no exogenous factors. Conditioned media (CM) from HS-5 promotes growth of myeloid colonies to significantly greater extent than a cocktail of recombinant factors containing 10 ng/mL of IL-1, IL-3, IL-6, G-CSF, GM-CSF, and KL and 3 U of erythropoietin (Epo). Two additional clones, HS-23 and HS-27, resemble blanket cells, with an epithelioid morphology, and are much larger, broader, and flatter when compared with HS-5 and HS-21. These lines secrete low levels of growth factors and do not support proliferation of isolated progenitor cells in cocultures. CM from HS-23 and HS-27 also fail to support growth of myeloid colonies. Both HS-23 and HS-27 express relatively high levels of VCAM-1, yet HS-27 is the only line that supports the formation of cobblestone areas by isolated CD34+38lo cells. We hypothesize that HS-5, HS-21, HS-23, and HS-27 represent functionally distinct components of the marrow microenvironment. | 7849321
 |