444966 Sigma-AldrichMEK1/2 Inhibitor III - CAS 391210-10-9 - Calbiochem
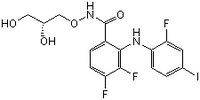
MEK1/2 Inhibitor III, CAS 391210-10-9, is a cell-permeable, non-competitive, highly potent MEK/MAPKK/MKK inhibitor that blocks cellular Erk1/2 phosphorylation (IC50 = 25 in serum starved HeLa cells).
More>> MEK1/2 Inhibitor III, CAS 391210-10-9, is a cell-permeable, non-competitive, highly potent MEK/MAPKK/MKK inhibitor that blocks cellular Erk1/2 phosphorylation (IC50 = 25 in serum starved HeLa cells). Less<<Synonyms: N-((2R)-2,3-Dihyroxypropoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)-benzamide, PD 0325901, PD0325901, PD325901, MEK Inhibitor III
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 391210-10-9 | C₁₆H₁₄F₃IN₂O₄ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 444966-5MG |
|
Plastic ampoule | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable benzamide compound that acts as a non-competitive, highly potent, selective MEK/MAPKK/MKK inhibitor and effectively blocks cellular Erk1/2 phosphorylation (by >90% in serum-starved HeLa after serum-stimulation and 50% in C26 with 25 and 0.33 nM inhibitor, respectively), while exhibiting little or no effect against the activity of Erk1/2 or a panel of 66 other kinases even at concentrations as high as 10 µM in cell-free kinase assays. Shown to be orally available in mice and suppress both the growth of and Erk phosphorylation in BRAF(V600E)-expressing SKMEL28 cell-derived tumor in vivo. Also available in InSolution™ format (Cat. No. 444968). |
| Catalogue Number | 444966 |
| Brand Family | Calbiochem® |
| Synonyms | N-((2R)-2,3-Dihyroxypropoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)-benzamide, PD 0325901, PD0325901, PD325901, MEK Inhibitor III |
| Product Information | |
|---|---|
| CAS number | 391210-10-9 |
| Form | White to off-white solid |
| Hill Formula | C₁₆H₁₄F₃IN₂O₄ |
| Chemical formula | C₁₆H₁₄F₃IN₂O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 444966-5MG | 04055977204520 |
Documentation
MEK1/2 Inhibitor III - CAS 391210-10-9 - Calbiochem MSDS
| Title |
|---|
MEK1/2 Inhibitor III - CAS 391210-10-9 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 444966 |
References
| Reference overview |
|---|
| Barrett, S.D., et al. 2008. Bioorg. Med. Chem. Lett. 18, 6501. Leyton, J., et al. 2008. Mol. Cancer Ther. 7, 3112. Silva, J., et al. 2008. PLoS Biol. 6, 2237. Ying, Q.L., et al. 2008. Nature 453, 519. Bain, J., et al. 2007. Biochem. J. 408, 297. Solit, D.B., et al. 2006. Nature 439, 358. |
Data Sheet
| Title |
|---|
| Reprogramming Cell Fate and Function Novel Strategies for iPSC Generation, Characterization, and Differentiation |