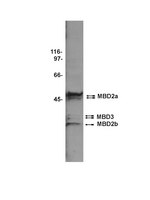
The methylated-DNA binding protein MBD2 enhances NGFI-A (egr-1)-mediated transcriptional activation of the glucocorticoid receptor.
Weaver, IC; Hellstrom, IC; Brown, SE; Andrews, SD; Dymov, S; Diorio, J; Zhang, TY; Szyf, M; Meaney, MJ
Philosophical transactions of the Royal Society of London. Series B, Biological sciences
369
2014
Show Abstract
Variations in maternal care in the rat influence the epigenetic state and transcriptional activity of glucocorticoid receptor (GR) gene in the hippocampus. The mechanisms underlying this maternal effect remained to be defined, including the nature of the relevant maternally regulated intracellular signalling pathways. We show here that increased maternal licking/grooming (LG), which stably enhances hippocampal GR expression, paradoxically increases hippocampal expression of the methyl-CpG binding domain protein-2 (MBD2) and MBD2 binding to the exon 17 GR promoter. Knockdown experiments of MBD2 in hippocampal primary cell culture show that MBD2 is required for activation of exon 17 GR promoter. Ectopic co-expression of nerve growth factor-inducible protein A (NGFI-A) with MBD2 in HEK 293 cells with site-directed mutagenesis of the NGFI-A response element within the methylated exon 17 GR promoter supports the hypothesis that MBD2 collaborates with NGFI-A in binding and activation of this promoter. These data suggest a possible mechanism linking signalling pathways, which are activated by behavioural stimuli and activation of target genes. | | 25135974
 |
MeCP2 knockdown reveals DNA methylation-independent gene repression of target genes in living cells and a bias in the cellular location of target gene products.
Shinya Yakabe,Hidenobu Soejima,Hitomi Yatsuki,Hirotaka Tominaga,Wei Zhao,Ken Higashimoto,Keiichiro Joh,Shinichi Kudo,Kohji Miyazaki,Tsunehiro Mukai
Genes & genetic systems
83
2008
Show Abstract
MeCP2, a methyl-CpG binding domain (MBD) protein, is known to bind to methylated CpG sites via a conserved MBD, leading to transcriptional repression. However, studies in cell-free system for gene repression and MeCP2 binding have suggested that DNA methylation-independent repression also occurs in living cells. It has been difficult to characterize the target genes of MeCP2 because a limited number have been identified to date. In this context, we screened for MeCP2 target genes using knockdown (KD) experiments combined with microarray gene expression analyses. Of the 49 genes that showed a more than three-fold increase in expression in two independent KD experiments conducted with different siRNA sets, unexpectedly, half (24 genes) did not contain promoter CpG islands (CGIs). Of seven selected genes that did contain CGIs, only two were methylated at the CGI, bound MeCP2 before KD, and reduced MeCP2 after KD. For three, MeCP2 was observed to bind to the unmethylated CGI before KD, and for one MeCP2 was reduced after KD. Another two genes neither had DNA methylation nor bound MeCP2 before KD. Gene ontology analysis suggested that MeCP2 represses a certain group of genes. These results suggest that in addition to the canonical gene repression function, MeCP2 can repress gene expression by binding to unmethylated DNA in particular genes in living cells. | | 18506103
 |
Histone modifications silence the GATA transcription factor genes in ovarian cancer.
Caslini, C; Capo-chichi, CD; Roland, IH; Nicolas, E; Yeung, AT; Xu, XX
Oncogene
25
5446-61
2006
Show Abstract
Altered expression of GATA factors was found and proposed as the underlying mechanism for dedifferentiation in ovarian carcinogenesis. In particular, GATA6 is lost or excluded from the nucleus in 85% of ovarian tumors and GATA4 expression is absent in majority of ovarian cancer cell lines. Here, we evaluated their DNA and histone epigenetic modifications in five ovarian epithelial and carcinoma cell lines (human 'immortalized' ovarian surface epithelium (HIO)-117, HIO-114, A2780, SKOV3 and ES2). GATA4 and GATA6 gene silencing was found to correlate with hypoacetylation of histones H3 and H4 and loss of histone H3/lysine K4 tri-methylation at their promoters in all lines. Conversely, histone H3/lysine K9 di-methylation and HP1gamma association were not observed, excluding reorganization of GATA genes into heterochromatic structures. The histone deacetylase inhibitor trichostatin A, but not the DNA methylation inhibitor 5'-aza-2'-deoxycytidine, re-established the expression of GATA4 and/or GATA6 in A2780 and HIO-114 cells, correlating with increased histone H3 and H4 acetylation, histone H3 lysine K4 methylation and DNase I sensitivity at the promoters. Therefore, altered histone modification of the promoter loci is one mechanism responsible for the silencing of GATA transcription factors and the subsequent loss of a target gene, the tumor suppressor Disabled-2, in ovarian carcinogenesis. | | 16607277
 |
Active repression of methylated genes by the chromosomal protein MBD1.
Ng, H H, et al.
Mol. Cell. Biol., 20: 1394-406 (2000)
2000
Show Abstract
MBD1 belongs to a family of mammalian proteins that share a methyl-CpG binding domain. Previous work has shown that MBD1 binds to methylated sites in vivo and in vitro and can repress transcription from methylated templates in transcription extracts and in cultured cells. In the present study we established by several experimental criteria that, contrary to a previous report, MBD1 is not a component of the MeCP1 repressor complex. We identified a powerful transcriptional repression domain (TRD) at the C terminus of MBD1 that can actively repress transcription at a distance. Methylation-dependent repression in vivo depends on the presence of both the TRD and the methyl-CpG binding domain. The mechanism is likely to involve deacetylation, since the deacetylase inhibitor trichostatin A can overcome MBD1-mediated repression. Accordingly, we found that endogenous MBD1 is particularly concentrated at sites of centromeric heterochromatin, where acetylated histone H4 is deficient. Unlike MBD2 and MeCP2, MBD1 is not depleted by antibodies to the histone deacetylase HDAC1. Thus, the deacetylase-dependent pathway by which MBD1 actively silences methylated genes is likely to be different from that utilized by the methylation-dependent repressors MeCP1 and MeCP2. | | 10648624
 |