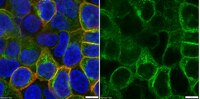
Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment.
Tan, XL; Xue, YQ; Ma, T; Wang, X; Li, JJ; Lan, L; Malik, KU; McDonald, MP; Dopico, AM; Liao, FF
Molecular neurodegeneration
10
24
2015
Show Abstract
Cerebral infarction due to thrombosis leads to the most common type of stroke and a likely cause of age-related cognitive decline and dementia. Endothelial nitric oxide synthase (eNOS) generates NO, which plays a crucial role in maintaining vascular function and exerting an antithrombotic action. Reduced eNOS expression and eNOS polymorphisms have been associated with stroke and Alzheimer's disease (AD), the most common type of dementia associated with neurovascular dysfunction. However, direct proof of such association is lacking. Since there are no reports of complete eNOS deficiency in humans, we used heterozygous eNOS(+/-) mice to mimic partial deficiency of eNOS, and determine its impact on cerebrovascular pathology and perfusion of cerebral vessels.Combining cerebral angiography with immunohistochemistry, we found thrombotic cerebral infarctions in eNOS(+/-) mice as early as 3-6 months of age but not in eNOS(+/+) mice at any age. Remarkably, vascular occlusions in eNOS(+/-) mice were found almost exclusively in three areas: temporoparietal and retrosplenial granular cortexes, and hippocampus this distribution precisely matching the hypoperfused areas identified in preclinical AD patients. Moreover, progressive cerebral amyloid angiopaphy (CAA), blood brain barrier (BBB) breakdown, and cognitive impairment were also detected in aged eNOS(+/-) mice.These data provide for the first time the evidence that partial eNOS deficiency results in spontaneous thrombotic cerebral infarctions that increase with age, leading to progressive CAA and cognitive impairments. We thus conclude that eNOS(+/-) mouse may represent an ideal model of ischemic stroke to address early and progressive damage in spontaneously-evolving chronic cerebral ischemia and thus, study vascular mechanisms contributing to vascular dementia and AD. | | | 26104027
 |
Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth.
Zhou, W; Ke, SQ; Huang, Z; Flavahan, W; Fang, X; Paul, J; Wu, L; Sloan, AE; McLendon, RE; Li, X; Rich, JN; Bao, S
Nature cell biology
17
170-82
2015
Show Abstract
Tumour-associated macrophages (TAMs) are enriched in glioblastoma multiformes (GBMs) that contain glioma stem cells (GSCs) at the apex of their cellular hierarchy. The correlation between TAM density and glioma grade suggests a supportive role for TAMs in tumour progression. Here we interrogated the molecular link between GSCs and TAM recruitment in GBMs and demonstrated that GSCs secrete periostin (POSTN) to recruit TAMs. TAM density correlates with POSTN levels in human GBMs. Silencing POSTN in GSCs markedly reduced TAM density, inhibited tumour growth, and increased survival of mice bearing GSC-derived xenografts. We found that TAMs in GBMs are not brain-resident microglia, but mainly monocyte-derived macrophages from peripheral blood. Disrupting POSTN specifically attenuated the tumour-supportive M2 type of TAMs in xenografts. POSTN recruits TAMs through the integrin αvβ₃ as blocking this signalling by an RGD peptide inhibited TAM recruitment. Our findings highlight the possibility of improving GBM treatment by targeting POSTN-mediated TAM recruitment. | | | 25580734
 |
The role of Hypoxia-inducible factor-1 α , glucose transporter-1, (GLUT-1) and carbon anhydrase IX in endometrial cancer patients.
Sadlecki, P; Bodnar, M; Grabiec, M; Marszalek, A; Walentowicz, P; Sokup, A; Zegarska, J; Walentowicz-Sadlecka, M
BioMed research international
2014
616850
2014
Show Abstract
Hypoxia-inducible factor-1α (HIF-1α), glucose transporter-1 (GLUT-1), and carbon anhydrase IX (CAIX) are important molecules that allow adaptation to hypoxic environments. The aim of our study was to investigate the correlation between HIF-1α, GLUT-1, and CAIX protein level with the clinicopathological features of endometrial cancer patients. Materials and Methods. 92 endometrial cancer patients, aged 37-84, were enrolled to our study. In all patients clinical stage, histologic grade, myometrial invasion, lymph node, and distant metastases were determined. Moreover, the survival time was assessed. Immunohistochemical analyses were performed on archive formalin fixed paraffin embedded tissue sections. Results. High significant differences (P = 0.0115) were reported between HIF-1α expression and the histologic subtype of cancer. Higher HIF-1α expression was associated with the higher risk of recurrence (P = 0.0434). The results of GLUT-1 and CAIX expression did not reveal any significant differences between the proteins expression in the primary tumor and the clinicopathological features. Conclusion. The important role of HIF-1α in the group of patients with the high risk of recurrence and the negative histologic subtype of the tumor suggest that the expression of this factor might be useful in the panel of accessory pathomorphological tests and could be helpful in establishing more accurate prognosis in endometrial cancer patients. | Immunohistochemistry (Paraffin) | Human | 24745019
 |
A conditional mouse mutant in the tumor suppressor SdhD gene unveils a link between p21(WAF1/Cip1) induction and mitochondrial dysfunction.
Millán-Uclés, A; Díaz-Castro, B; García-Flores, P; Báez, A; Pérez-Simón, JA; López-Barneo, J; Piruat, JI
PloS one
9
e85528
2014
Show Abstract
Mutations in mitochondrial complex II (MCII; succinate dehydrogenase, Sdh) genes cause familiar pheochromocytoma/paraganglioma tumors. Several mechanisms have been proposed to account for Sdh-mutation-induced tumorigenesis, the most accepted of which is based on the constitutive expression of the hypoxia-inducible factor 1α (Hif1α) at normal oxygen tension, a theory referred to as "pseudo-hypoxic drive". Other molecular processes, such as oxidative stress, apoptosis, or chromatin remodeling have been also proposed to play a causative role. Nevertheless, the actual contribution of each of these mechanisms has not been definitively established. Moreover, the biological factors that determine the tissue-specificity of these tumors have not been identified. In this work, we made use of the inducible SDHD-ESR mouse, a conditional mutant in the SdhD gene, which encodes the small subunit of MCII, and that acts as a tumor suppressor gene in humans. The analysis of the Hif1α pathway in SDHD-ESR tissues and in two newly derived cell lines after complete SdhD loss -a requirement for hereditary paraganglioma type-1 tumor formation in humans- partially recapitulated the "pseudo-hypoxic" response and rendered inconsistent results. Therefore, we performed microarray analysis of adrenal medulla and kidney in order to identify other early gene expression changes elicited by SdhD deletion. Our results revealed that each mutant tissue displayed different variations in their gene expression profiles affecting to different biological processes. However, we found that the Cdkn1a gene was up-regulated in both tissues. This gene encodes the cyclin-dependent kinase inhibitor p21(WAF1/Cip1), a factor implicated in cell cycle, senescence, and cancer. The two SDHD-ESR cell lines also showed accumulation of this protein. This new and unprecedented evidence for a link between SdhD dysfunction and p21(WAF1/Cip1) will open new avenues for the study of the mechanisms that cause tumors in Sdh mutants. Finally, we discuss the actual role of Hif1α in tumorigenesis. | Western Blotting | | 24465590
 |
Hyperglycemia promotes K-Ras-induced lung tumorigenesis through BASCs amplification.
Micucci, C; Orciari, S; Catalano, A
PloS one
9
e105550
2014
Show Abstract
Oncogenic K-Ras represents the most common molecular change in human lung adenocarcinomas, the major histologic subtype of non-small cell lung cancer (NSCLC). The presence of K-Ras mutation is associated with a poor prognosis, but no effective treatment strategies are available for K-Ras -mutant NSCLC. Epidemiological studies report higher lung cancer mortality rates in patients with type 2 diabetes. Here, we use a mouse model of K-Ras-mediated lung cancer on a background of chronic hyperglycemia to determine whether elevated circulating glycemic levels could influence oncogenic K-Ras-mediated tumor development. Inducible oncogenic K-Ras mouse model was treated with subtoxic doses of streptozotocin (STZ) to induce chronic hyperglycemia. We observed increased tumor mass and higher grade of malignancy in STZ treated diabetic mice analyzed at 4, 12 and 24 weeks, suggesting that oncogenic K-Ras increased lung tumorigenesis in hyperglycemic condition. This promoting effect is achieved by expansion of tumor-initiating lung bronchio-alveolar stem cells (BASCs) in bronchio-alveolar duct junction, indicating a role of hyperglycemia in the activity of K-Ras-transformed putative lung stem cells. Notably, after oncogene K-Ras activation, BASCs show upregulation of the glucose transporter (Glut1/Slc2a1), considered as an important player of the active control of tumor cell metabolism by oncogenic K-Ras. Our novel findings suggest that anti-hyperglycemic drugs, such as metformin, may act as therapeutic agent to restrict lung neoplasia promotion and progression. | | | 25144301
 |
Palmitoleic acid (n-7) increases white adipocytes GLUT4 content and glucose uptake in association with AMPK activation.
Bolsoni-Lopes, A; Festuccia, WT; Chimin, P; Farias, TS; Torres-Leal, FL; Cruz, MM; Andrade, PB; Hirabara, SM; Lima, FB; Alonso-Vale, MI
Lipids in health and disease
13
199
2014
Show Abstract
Palmitoleic acid was previously shown to improve glucose homeostasis by reducing hepatic glucose production and by enhancing insulin-stimulated glucose uptake in skeletal muscle. Herein we tested the hypothesis that palmitoleic acid positively modulates glucose uptake and metabolism in adipocytes.For this, both differentiated 3 T3-L1 cells treated with either palmitoleic acid (16:1n7, 200 μM) or palmitic acid (16:0, 200 μM) for 24 h and primary adipocytes from mice treated with 16:1n7 (300 mg/kg/day) or oleic acid (18:1n9, 300 mg/kg/day) by gavage for 10 days were evaluated for glucose uptake, oxidation, conversion to lactate and incorporation into fatty acids and glycerol components of TAG along with the activity and expression of lipogenic enzymes.Treatment of adipocytes with palmitoleic, but not oleic (in vivo) or palmitic (in vitro) acids, increased basal and insulin-stimulated glucose uptake and GLUT4 mRNA levels and protein content. Along with uptake, palmitoleic acid enhanced glucose oxidation (aerobic glycolysis), conversion to lactate (anaerobic glycolysis) and incorporation into glycerol-TAG, but reduced de novo fatty acid synthesis from glucose and acetate and the activity of lipogenic enzymes glucose 6-phosphate dehydrogenase and ATP-citrate lyase. Importantly, palmitoleic acid induction of adipocyte glucose uptake and metabolism were associated with AMPK activation as evidenced by the increased protein content of phospho(p)Thr172AMPKα, but no changes in pSer473Akt and pThr308Akt. Importantly, such increase in GLUT4 content induced by 16:1n7, was prevented by pharmacological inhibition of AMPK with compound C.In conclusion, palmitoleic acid increases glucose uptake and the GLUT4 content in association with AMPK activation. | Western Blotting | | 25528561
 |
Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia.
Herzog, RI; Jiang, L; Herman, P; Zhao, C; Sanganahalli, BG; Mason, GF; Hyder, F; Rothman, DL; Sherwin, RS; Behar, KL
The Journal of clinical investigation
123
1988-98
2013
Show Abstract
Hypoglycemia occurs frequently during intensive insulin therapy in patients with both type 1 and type 2 diabetes and remains the single most important obstacle in achieving tight glycemic control. Using a rodent model of hypoglycemia, we demonstrated that exposure to antecedent recurrent hypoglycemia leads to adaptations of brain metabolism so that modest increments in circulating lactate allow the brain to function normally under acute hypoglycemic conditions. We characterized 3 major factors underlying this effect. First, we measured enhanced transport of lactate both into as well as out of the brain that resulted in only a small increase of its contribution to total brain oxidative capacity, suggesting that it was not the major fuel. Second, we observed a doubling of the glucose contribution to brain metabolism under hypoglycemic conditions that restored metabolic activity to levels otherwise only observed at euglycemia. Third, we determined that elevated lactate is critical for maintaining glucose metabolism under hypoglycemia, which preserves neuronal function. These unexpected findings suggest that while lactate uptake was enhanced, it is insufficient to support metabolism as an alternate substrate to replace glucose. Lactate is, however, able to modulate metabolic and neuronal activity, serving as a "metabolic regulator" instead. | | | 23543056
 |
Impact of tumor vascularity on responsiveness to antiangiogenesis in a prostate cancer stem cell-derived tumor model.
Zhang, K; Waxman, DJ
Molecular cancer therapeutics
12
787-98
2013
Show Abstract
Drugs that target the tumor vasculature and inhibit angiogenesis are widely used for cancer treatment. Individual tumors show large differences in vascularity, but it is uncertain how these differences affect responsiveness to antiangiogenesis. We investigated this question using two closely related prostate cancer models that differ markedly in tumor vascularity: PC3, which has very low vascularity, and the PC3-derived cancer stem-like cell holoclone PC3/2G7, which forms tumors with high microvessel density, high tumor blood flow, and low hypoxia compared with parental PC3 tumors. Three angiogenesis inhibitors (axitinib, sorafenib, and DC101) all induced significantly greater decreases in tumor blood flow and microvessel density in PC3/2G7 tumors compared with PC3 tumors, as well as significantly greater decreases in tumor cell proliferation and cell viability and a greater increase in apoptosis. The increased sensitivity of PC3/2G7 tumors to antiangiogenesis indicates they are less tolerant of low vascularity and suggests they become addicted to their oxygen- and nutrient-rich environment. PC3/2G7 tumors showed strong upregulation of the proangiogenic factors chemokine ligand 2 (CCL2) and VEGFA compared with PC3 tumors, which may contribute to their increased vascularity, and they have significantly lower endothelial cell pericyte coverage, which may contribute to their greater sensitivity to antiangiogenesis. Interestingly, high levels of VEGF receptor-2 were expressed on PC3 but not PC3/2G7 tumor cells, which may contribute to the growth static response of PC3 tumors to VEGF-targeted antiangiogenesis. Finally, prolonged antiangiogenic treatment led to resumption of PC3/2G7 tumor growth and neovascularization, indicating these cancer stem-like cell-derived tumors can adapt and escape from antiangiogenesis. | Immunohistochemistry | | 23635653
 |
A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-α.
Begley, U; Sosa, MS; Avivar-Valderas, A; Patil, A; Endres, L; Estrada, Y; Chan, CT; Su, D; Dedon, PC; Aguirre-Ghiso, JA; Begley, T
EMBO molecular medicine
5
366-83
2013
Show Abstract
Emerging evidence points to aberrant regulation of translation as a driver of cell transformation in cancer. Given the direct control of translation by tRNA modifications, tRNA modifying enzymes may function as regulators of cancer progression. Here, we show that a tRNA methyltransferase 9-like (hTRM9L/KIAA1456) mRNA is down-regulated in breast, bladder, colorectal, cervix and testicular carcinomas. In the aggressive SW620 and HCT116 colon carcinoma cell lines, hTRM9L is silenced and its re-expression and methyltransferase activity dramatically suppressed tumour growth in vivo. This growth inhibition was linked to decreased proliferation, senescence-like G0/G1-arrest and up-regulation of the RB interacting protein LIN9. Additionally, SW620 cells re-expressing hTRM9L did not respond to hypoxia via HIF1-α-dependent induction of GLUT1. Importantly, hTRM9L-negative tumours were highly sensitive to aminoglycoside antibiotics and this was associated with altered tRNA modification levels compared to antibiotic resistant hTRM9L-expressing SW620 cells. Our study links hTRM9L and tRNA modifications to inhibition of tumour growth via LIN9 and HIF1-α-dependent mechanisms. It also suggests that aminoglycoside antibiotics may be useful to treat hTRM9L-deficient tumours. | Immunocytochemistry | | 23381944
 |
Fibronectin extra domain A (EDA) sustains CD133(+)/CD44(+) subpopulation of colorectal cancer cells.
Ou, J; Deng, J; Wei, X; Xie, G; Zhou, R; Yu, L; Liang, H
Stem cell research
11
820-33
2013
Show Abstract
Fibronectin is a major extracellular matrix glycoprotein with several alternatively spliced variants, including extra domain A (EDA), which was demonstrated to promote tumorigenesis via stimulating angiogenesis and lymphangiogenesis. Given that CD133(+)/CD44(+) cancer cells are critical in tumorigenesis of colorectal cancer (CRC), we hypothesize that fibronectin EDA may promote tumorigenesis by sustaining the properties of CD133(+)/CD44(+) colon cancer cells. We found that tumor tissue and serum EDA levels are substantially higher in advanced versus early stage human CRC. Additionally we showed that tumor tissue EDA levels are positively correlated with differentiation status and chemoresistance, and correlated with a poor prognosis of CRC patients. We also showed that in colon cancer cells SW480, CD133(+)/CD44(+) versus CD133(-)/CD44(-) cells express significantly elevated EDA receptor integrin α9β1. Silencing EDA in SW480 cells reduces spheroid formation and cells positive for CD133 or CD44, which is associated with reduced expressions of embryonic stem cell markers and increased expressions of differentiation markers. Blocking integrin α9β1 function strongly reversed the effect of EDA overexpression. We also provided evidence suggesting that EDA sustains Wnt/β-catenin signaling activity via activating integrin/FAK/ERK pathway. In xenograft models, EDA-silenced SW480 cells exhibit reduced tumorigenic and metastatic capacity. In conclusion, EDA is essential for the maintenance of the properties of CD133(+)/CD44(+) colon cancer cells. | | | 23811539
 |