182540 Sigma-AldrichAromatase Inhibitor I - CAS 331684-05-0 - Calbiochem
The Aromatase Inhibitor I, also referenced under CAS 331684-05-0, controls the biological activity of Aromatase.
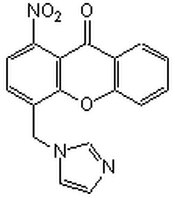
More>> The Aromatase Inhibitor I, also referenced under CAS 331684-05-0, controls the biological activity of Aromatase. Less<<Synonyms: 4-(Imidazolylmethyl)-1-nitro-9H-9-xanthenone
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 331684-05-0 | C₁₇H₁₁N₃O₄ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 182540-1MG |
|
Plastic ampoule | 1 mg |
|
— |
| References | |
|---|---|
| References | Recanatini, M., et al. 2001. J. Med. Chem. 44, 672. |
| Product Information | |
|---|---|
| CAS number | 331684-05-0 |
| ATP Competitive | Y |
| Form | White solid |
| Hill Formula | C₁₇H₁₁N₃O₄ |
| Chemical formula | C₁₇H₁₁N₃O₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | P450 arom. |
| Primary Target IC<sub>50</sub> | 40 nM against P450arom |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 182540-1MG | 04055977205091 |
Documentation
Aromatase Inhibitor I - CAS 331684-05-0 - Calbiochem MSDS
| Title |
|---|
Aromatase Inhibitor I - CAS 331684-05-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 182540 |
References
| Reference overview |
|---|
| Recanatini, M., et al. 2001. J. Med. Chem. 44, 672. |