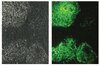
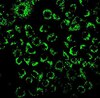
CBA043 Sigma-AldrichInnoCyte™ Laminin-based 96-Well Cell Invasion Assay
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Detection Methods |
|---|
| Fluorometric |
| Applications | |
|---|---|
| Application References | Caroll, D.K., et al. 2006. Nat. Cell Biol. 8, 551. |
| Biological Information | |
|---|---|
| Assay time | 14-26 h |
| Sample Type | Cells |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information | |
|---|---|
| Kit contains | 96-Well Laminin-Coated 96-Well Cell Invasion Chamber, Black 96-Well Plate, Additional Tray , Calcein-AM Solution, Cell Detachment Buffer, and a user protocol. |
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| CBA043 | 0 |
Documentation
InnoCyte™ Laminin-based 96-Well Cell Invasion Assay Certificates of Analysis
| Title | Lot Number |
|---|---|
| CBA043 |
References
| Reference overview |
|---|
| Caroll, D.K., et al. 2006. Nat. Cell Biol. 8, 551. Ekblom, P., et al. 2003. Matrix Biol. 22, 35. Hood, J.D. and Cheresh, D.A. 2002. Nat. Rev. Cancer 2, 91. Aumailley, M., et al. 1998. J. Anat. 193, 1. Aumailley, M., et al. 1996. J. Invest. Dermatol. 106, 209. Burgeson, et al. 1994, Matrix Biol. 14, 209. Aznavoorian, S., et al. 1993. Cancer 71, 1368. Stetler-Stevenson, W. G., et al. 1993. Annu. Rev. Cell Biol. 9, 541. |
Brochure
| Title |
|---|
| Kit SourceBook - 2nd Edition EURO |