Saturation mutagenesis of the plasminogen activator inhibitor-1 reactive center.
Sherman, P M, et al.
J. Biol. Chem., 267: 7588-95 (1992)
1992
Show Abstract
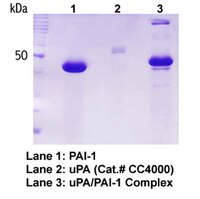
Plasminogen activator inhibitor-1 (PAI-1) is a specific inhibitor of the serine proteases tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). To systematically investigate the roles of the reactive center P1 and P1' residues in PAI-1 function, saturation mutagenesis was utilized to construct a library of PAI-1 variants. Examination of 177 unique recombinant proteins indicated that a basic residue was required at P1 for significant inhibitory activity toward uPA, whereas all substitutions except proline were tolerated at P1'. P1Lys variants exhibited lower inhibition rate constants and greater sensitivity to P1' substitutions than P1Arg variants. Alterations at either P1 or P1' generally had a larger effect on the inhibition of tPA. A number of variants that were relatively specific for either uPA or tPA were identified. P1Lys-P1'Ala reacted 40-fold more rapidly with uPA than tPA, whereas P1Lys-P1'Trp showed a 6.5-fold preference for tPA. P1-P1' variants containing additional mutations near the reactive center demonstrated only minor changes in activity, suggesting that specific amino acids in this region do not contribute significantly to PAI-1 function. These findings have important implications for the role of reactive center residues in determining serine protease inhibitor (serpin) function and target specificity. | 1559996
 |