509583 Sigma-AldrichDiaphanous (mDia)-related Formin Agonist, IMM01 - CAS 218795-74-5
An agonist of mammalian Diaphanous (mDia)-related formins that disrupts diaphanous inhibitory domain (DID) and diaphanous autoregulatory domain (DAD) interaction (IC₅₀ = 140 nM).
More>> An agonist of mammalian Diaphanous (mDia)-related formins that disrupts diaphanous inhibitory domain (DID) and diaphanous autoregulatory domain (DAD) interaction (IC₅₀ = 140 nM). Less<<Synonyms: Formin Activator
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
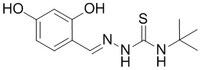
| 218795-74-5 | C₁₂H₁₇N₃O₂S |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.09583.0001 | Glass bottle | 10 mg |
| References | |
|---|---|
| References | Lash, L.L., et al. 2013. Cancer Res.73, 6793. Copeland, S.J. et al. 2007. J Biol. Chem. 282, 30120. |
| Product Information | |
|---|---|
| CAS number | 218795-74-5 |
| Form | Off-white powder |
| Hill Formula | C₁₂H₁₇N₃O₂S |
| Chemical formula | C₁₂H₁₇N₃O₂S |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | mDia |
| Primary Target IC<sub>50</sub> | 140 nM |
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.09583.0001 | 04055977240962 |
Documentation
Diaphanous (mDia)-related Formin Agonist, IMM01 - CAS 218795-74-5 SDS
| Title |
|---|
References
| Reference overview |
|---|
| Lash, L.L., et al. 2013. Cancer Res.73, 6793. Copeland, S.J. et al. 2007. J Biol. Chem. 282, 30120. |