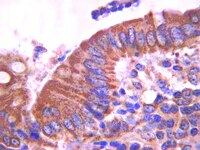
Human colonic crypts in culture: segregation of immunochemical markers in normal versus adenoma-derived.
Dame, MK; Jiang, Y; Appelman, HD; Copley, KD; McClintock, SD; Aslam, MN; Attili, D; Elmunzer, BJ; Brenner, DE; Varani, J; Turgeon, DK
Laboratory investigation; a journal of technical methods and pathology
94
222-34
2014
Show Abstract
In order to advance a culture model of human colonic neoplasia, we developed methods for the isolation and in vitro maintenance of intact colonic crypts from normal human colon tissue and adenomas. Crypts were maintained in three-dimensional Matrigel culture with a simple, serum-free, low Ca(2+) (0.15 mM) medium. Intact colonic crypts from normal human mucosa were viably maintained for 3-5 days with preservation of the in situ crypt-like architecture, presenting a distinct base and apex. Abnormal structures from adenoma tissue could be maintained through multiple passages (up to months), with expanding buds/tubules. Immunohistochemical markers for intestinal stem cells (Lgr5), growth (Ki67), differentiation (E-cadherin, cytokeratin 20 (CK20) and mucin 2 (MUC2)) and epithelial turnover (Bax, cleaved Caspase-3), paralleled the changes in function. The epithelial cells in normal crypts followed the physiological sequence of progression from proliferation to differentiation to dissolution in a spatially and temporally appropriate manner. Lgr5 expression was seen in a few basal cells of freshly isolated crypts, but was not detected after 1-3 days in culture. After 24 h in culture, crypts from normal colonic tissue continued to show strong Ki67 and MUC2 expression at the crypt base, with a gradual decrease over time such that by days 3-4 Ki67 was not expressed. The differentiation marker CK20 increased over the same period, eventually becoming intense throughout the whole crypt. In adenoma-derived structures, expression of markers for all stages of progression persisted for the entire time in culture. Lgr5 showed expression in a few select cells after months in culture. Ki67 and MUC2 were largely associated with the proliferative budding regions while CK20 was localized to the parent structure. This ex vivo culture model of normal and adenomatous crypts provides a readily accessible tool to help understand the growth and differentiation process in human colonic epithelium. | | 24365748
 |
The use of an in vitro 3D melanoma model to predict in vivo plasmid transfection using electroporation.
Marrero, B; Heller, R
Biomaterials
33
3036-46
2012
Show Abstract
A large-scale in vitro 3D tumor model was generated to evaluate gene delivery procedures in vivo. This 3D tumor model consists of a "tissue-like" spheroid that provides a micro-environment supportive of melanoma proliferation, allowing cells to behave similarly to cells in vivo. This functional spheroid measures approximately 1 cm in diameter and can be used to effectively evaluate plasmid transfection when testing various electroporation (EP) electrode applicators. In this study, we identified EP conditions that efficiently transfect green fluorescent protein (GFP) and interleukin 15 (IL-15) plasmids into tumor cells residing in the 3D construct. We found that plasmids delivered using a 6-plate electrode applying 6 pulses with nominal electric field strength of 500 V/cm and pulse-length of 20 ms produced significant increase of GFP (7.3-fold) and IL-15 (3.0-fold) expression compared to controls. This in vitro 3D model demonstrates the predictability of cellular response toward delivery techniques, limits the numbers of animals employed for transfection studies, and may facilitate future developments of clinical trials for cancer therapies in vivo. | | 22244695
 |
Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research.
Stewart, CE; Torr, EE; Mohd Jamili, NH; Bosquillon, C; Sayers, I
Journal of allergy
2012
943982
2012
Show Abstract
The aim of the current study was to evaluate primary (human bronchial epithelial cells, HBEC) and non-primary (Calu-3, BEAS-2B, BEAS-2B R1) bronchial epithelial cell culture systems as air-liquid interface- (ALI-) differentiated models for asthma research. Ability to differentiate into goblet (MUC5AC+) and ciliated (β-Tubulin IV+) cells was evaluated by confocal imaging and qPCR. Expression of tight junction/adhesion proteins (ZO-1, E-Cadherin) and development of transepithelial electrical resistance (TEER) were assessed. Primary cells showed localised MUC5AC, β-Tubulin IV, ZO-1, and E-Cadherin and developed TEER with, however, a large degree of inter- and intradonor variation. Calu-3 cells developed a more reproducible TEER and a phenotype similar to primary cells although with diffuse β-Tubulin IV staining. BEAS-2B cells did not differentiate or develop tight junctions. These data highlight the challenges in working with primary cell models and the need for careful characterisation and selection of systems to answer specific research questions. | | 22287976
 |
Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes.
Caberg, Jean-Hubert D, et al.
Carcinogenesis, 29: 1441-7 (2008)
2008
Show Abstract
Human papillomavirus (HPV) infection, particularly type 16, is causally associated with cancer of the uterine cervix. The persistence or progression of cervical lesions suggests that viral antigens are not adequately presented to the immune system. This hypothesis is reinforced by the observation that most squamous intra-epithelial lesions show quantitative and functional alterations of Langerhans cells (LCs). Moreover, E-cadherin-dependent adhesion of LC to keratinocytes (KCs) is defective in cervical HPV16-associated (pre)neoplastic lesions. The possible role of viral oncoprotein E7 in the reduced levels of cell surface E-cadherin was investigated by silencing HPV16 E7 by RNA interference (siRNA). This treatment induced an increased cell surface E-cadherin expression in HPV16-positive KC and a significant adhesion of LC to these squamous cells. The E-cadherin re-expression following HPV16 E7 silencing was associated with increased detection levels of retinoblastoma protein and the activating protein (AP)-2alpha transcription factor. These data suggest that HPV16 E7-induced alterations of LC/KC adhesion may play a role in the defective immune response during cervical carcinogenesis. | | 18566017
 |
Controlled, scalable embryonic stem cell differentiation culture.
Dang, Stephen M, et al.
Stem Cells, 22: 275-82 (2004)
2004
Show Abstract
Embryonic stem (ES) cells are of significant interest as a renewable source of therapeutically useful cells. ES cell aggregation is important for both human and mouse embryoid body (EB) formation and the subsequent generation of ES cell derivatives. Aggregation between EBs (agglomeration), however, inhibits cell growth and differentiation in stirred or high-cell-density static cultures. We demonstrate that the agglomeration of two EBs is initiated by E-cadherin-mediated cell attachment and followed by active cell migration. We report the development of a technology capable of controlling cell-cell interactions in scalable culture by the mass encapsulation of ES cells in size-specified agarose capsules. When placed in stirred-suspension bioreactors, encapsulated ES cells can be used to produce scalable quantities of hematopoietic progenitor cells in a controlled environment. | | 15153605
 |
E-cadherin-mediated interactions of thymic epithelial cells with CD103+ thymocytes lead to enhanced thymocyte cell proliferation.
Kutlesa, Snjezana, et al.
J. Cell. Sci., 115: 4505-15 (2002)
2002
Show Abstract
Cadherins are a family of cell adhesion molecules that mainly mediate homotypic homophilic interactions, but for E-cadherin, heterophilic interactions with the integrin alpha(E)(CD103)beta(7) have also been reported. In the human thymus, where thymocytes develop in close contact with thymic stromal cells, E-cadherin expression was detected on thymic epithelial cells. By immunofluorescence staining, the strongest expression of E-cadherin was observed on medullary thymic epithelial cells. These cells also express cytosolic catenins, which are necessary to form functional cadherin-catenin complexes. Regardless of their developmental stage, human thymocytes do not express E-cadherin, indicating that homophilic interactions cannot occur. Flow cytometric analysis revealed that the E-cadherin ligand CD103 is expressed on subpopulations of the early CD4(-) CD8(-) double-negative and of the more mature CD8(+) single-positive thymocytes. Using an in vitro cell adhesion assay, double-negative and CD8(+) single-positive thymocytes adhered strongly to isolated thymic epithelial cells. These adhesive interactions could be inhibited by antibodies against E-cadherin or CD103. CD8(+) thymocytes showed a proliferative response when incubated with thymic epithelial cells. This mitogenic effect was inhibited by antibodies against CD103, which strongly indicates a direct involvement of the adhesive ligand pair CD103-E-cadherin in human thymocyte cell proliferation. | Immunoblotting (Western) | 12414996
 |
The adhesion molecule E-cadherin and a surface antigen recognized by the antibody 9C4 are selectively expressed on erythroid cells of defined maturational stages.
Bühring, H J, et al.
Leukemia, 10: 106-16 (1996)
1996
Show Abstract
The antigen expression of immature erythroid bone marrow cells was studied using two recently generated monoclonal antibodies (mAb), mAb 67A4 and 9C4, with specificities for the epithelial cell adhesion molecule E-cadherin (E-cad; mAb 67A4), and a novel 110 kDa differentiation antigen (mAb 9C4) with unknown molecular structure. Pappenheim staining of FACS-purified cells labeled with mAb 9C4 and anti-glycophorin A (GA) revealed that the majority of the 9C4+GA- and 9C4+GA+ cells consisted of erythroblasts. In contrast, the E-cad-positive population comprised normoblasts and erythroblasts. While the E-cad+GA- fraction contained mainly erythroblasts and basophilic normoblasts, the E-Cad+GA+ population was enriched in orthochromatic and polychromatophilic normoblasts. By colony assays of affinity column-purified cells it could be shown that erythroid colony forming units (CFU-E) were enriched and erythroid burst forming units (BFU-E) were depleted in the 9C4- and E-cad-positive fractions. Flow cytometric analysis of bone marrow cells double-labeled with mAb 67A4 and anti-CD71, anti-CD117, anti-CD34, or anti-GA revealed that about 90% of the E-cad-positive cells coexpressed CD71, about 70% were positive for CD117, about 50% for GA, and only about 5% coexpressed CD34. The expression pattern of 9C4 antigen was similar to that of E-Cad with the exception that only a minority of the 9C4-positive cells coexpressed GA. Lymphoid and myeloid markers were negative on both the E-Cad- and 9C4-positive populations. In these studies we describe the identification of a new mAb-defined antigen which is specifically expressed on erythroblasts and CFU-E(9C4) and demonstrate that E-Cad is not only expressed on epithelial cells but also on erythropoietic cells of defined maturational stages. | | 8558914
 |