208925 Sigma-AldrichCamptothecin, Camptotheca acuminata - CAS 2114454 - Calbiochem
A cell-permeable DNA topoisomerase I inhibitor.
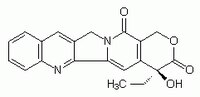
More>> A cell-permeable DNA topoisomerase I inhibitor. Less<<Synonyms: 4-Ethyl-4-hydroxy-1H-pyrano[3ʹ,4ʹ:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)dione
Recommended Products
Przegląd
| Replacement Information |
|---|
Tabela kluczowych gatunków
| CAS # | Empirical Formula |
|---|---|
| 7689-03-4 | C₂₀H₁₆N₂O₄ |
Products
| Numer katalogowy | Opakowanie | Ilość/opak. | |
|---|---|---|---|
| 208925-50MG | Beben aluminiowy | 50 mg |
| Product Information | |
|---|---|
| CAS number | 7689-03-4 |
| ATP Competitive | N |
| Form | Pale yellow solid |
| Hill Formula | C₂₀H₁₆N₂O₄ |
| Chemical formula | C₂₀H₁₆N₂O₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | DNA topoisomerase 1 |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | UQ0492000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numer katalogowy | GTIN |
| 208925-50MG | 04055977219128 |
Documentation
Camptothecin, Camptotheca acuminata - CAS 2114454 - Calbiochem MSDS
| Title |
|---|
Camptothecin, Camptotheca acuminata - CAS 2114454 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 208925 |
References
| Przegląd literatury |
|---|
| Jones, C.B., et al. 1997. Cancer Chemother. Pharmacol. 40, 475. Staron, K., et al. 1994. Carcinogenesis 15, 2953. Tanizawa, A., et al. 1994. J. Natl. Cancer Inst. 86, 836. Gorczyca, W., et al. 1993. Toxicol. Lett. 67, 249. Onishi, Y., et al. 1993. Biochim. Biophys. Acta 1175, 147. Pantazis, P., et al. 1993. Int. J. Cancer 53, 863. Morham, S.G., and Shuman, S. 1992. J. Biol. Chem. 267, 15984. Hertzberg, R.P., et al. 1990. Biochem. J. 28, 4629. Hertzberg, R.P., et al. 1990. J. Biol. Chem. 265, 19287. Hsiang, Y.H., et al. 1985. J. Biol. Chem. 260, 14873. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
| Tools and Tips for Analyzing Apoptosis |