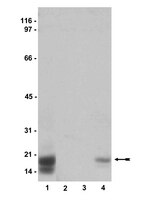
Increased PDE5 activity and decreased Rho kinase and PKC activities in colonic muscle from caveolin-1-/- mice impair the peristaltic reflex and propulsion.
Mahavadi, S; Bhattacharya, S; Kumar, DP; Clay, C; Ross, G; Akbarali, HI; Grider, JR; Murthy, KS
American journal of physiology. Gastrointestinal and liver physiology
305
G964-74
2013
Pokaż streszczenie
Caveolae are specialized regions of the plasma membrane that concentrate receptors and associated signaling molecules critical in regulation of cellular response to transmitters and hormones. We have determined the effects of caveolin-1 (Cav-1) deletion, caveolin-1 siRNA, and caveolar disruption in mice on the signaling pathways that mediate contraction and relaxation in colonic smooth muscle and on the components of the peristaltic reflex in isolated tissue and propulsion in intact colonic segments. In Cav-1-/- mice, both relaxation and contraction were decreased in smooth muscle cells and muscle strips, as well as during both phases of the peristaltic reflex and colonic propulsion. The decrease in relaxation in response to the nitric oxide (NO) donor was accompanied by a decrease in cGMP levels and an increase in phosphodiesterase 5 (PDE5) activity. Relaxation by a PDE5-resistant cGMP analog was not affected in smooth muscle of Cav-1-/- mice, suggesting that inhibition of relaxation was due to augmentation of PDE5 activity. Similar effects on relaxation, PDE5 and cGMP were obtained in muscle cells upon disruption of caveolae by methyl-β-cyclodextrin or suppression of Cav-1. Sustained contraction mediated via inhibition of myosin light chain phosphatase (MLCP) activity is regulated by Rho kinase and PKC via phosphorylation of two endogenous inhibitors of MLCP: myosin phosphatase-targeting subunit (MYPT1) and 17-kDa PKC-potentiated protein phosphatase 1 inhibitor protein (CPI-17), respectively. The activity of both enzymes and phosphorylation of MYPT1 and CPI-17 were decreased in smooth muscle from Cav-1-/- mice. We conclude that the integrity of caveolae is essential for contractile and relaxant activity in colonic smooth muscle and the maintenance of neuromuscular function at organ level. | | | 24157969
 |
Altered calcium signaling in colonic smooth muscle of type 1 diabetic mice.
Touw, K; Chakraborty, S; Zhang, W; Obukhov, AG; Tune, JD; Gunst, SJ; Herring, BP
American journal of physiology. Gastrointestinal and liver physiology
302
G66-76
2011
Pokaż streszczenie
Seventy-six percent of diabetic patients develop gastrointestinal symptoms, such as constipation. However, the direct effects of diabetes on intestinal smooth muscle are poorly described. This study aimed to identify the role played by smooth muscle in mediating diabetes-induced colonic dysmotility. To induce type 1 diabetes, mice were injected intraperitoneally with low-dose streptozotocin once a day for 5 days. Animals developed hyperglycemia (greater than 200 mg/dl) 1 wk after the last injection and were euthanized 7-8 wk after the last treatment. Computed tomography demonstrated decreased overall gastrointestinal motility in the diabetic mice. In vitro contractility of colonic smooth muscle rings from diabetic mice was also decreased. Fura-2 ratiometric Ca(2+) imaging showed attenuated Ca(2+) increases in response to KCl stimulation that were associated with decreased light chain phosphorylation in diabetic mice. The diabetic mice also exhibited elevated basal Ca(2+) levels, increased myosin phosphatase targeting subunit 1 expression, and significant changes in expression of Ca(2+) handling proteins, as determined by quantitative RT-PCR and Western blotting. Mice that were hyperglycemic for less than 1 wk also showed decreased colonic contractile responses that were associated with decreased Ca(2+) increases in response to KCl stimulation, although without an elevation in basal Ca(2+) levels or a significant change in the expression of Ca(2+) signaling molecules. These data demonstrate that type 1 diabetes is associated with decreased depolarization-induced Ca(2+) influx in colonic smooth muscle that leads to attenuated myosin light chain phosphorylation and impaired colonic contractility. | | | 21979758
 |
Ca2+-independent, inhibitory effects of cyclic adenosine 5'-monophosphate on Ca2+ regulation of phosphoinositide 3-kinase C2alpha, Rho, and myosin phosphatase in vascular smooth muscle.
Azam, MA; Yoshioka, K; Ohkura, S; Takuwa, N; Sugimoto, N; Sato, K; Takuwa, Y
The Journal of pharmacology and experimental therapeutics
320
907-16
2007
Pokaż streszczenie
We have recently demonstrated in vascular smooth muscle (VSM) that membrane depolarization by high KCl induces Ca(2+)-dependent Rho activation and myosin phosphatase (MLCP) inhibition (Ca(2+)-induced Ca(2+)-sensitization) through the mechanisms involving phosphorylation of myosin-targeting protein 1 (MYPT1) and 17-kDa protein kinase C (PKC)-potentiated inhibitory protein of PP1 (CPI-17). In the present study, we investigated whether and how cAMP affected Ca(2+)-dependent MLCP inhibition by examining the effects of forskolin, cell-permeable dibutyryl cAMP (dbcAMP), and isoproterenol. Forskolin, but not its inactive analog 1,9-dideoxyforskolin, inhibited KCl-induced contraction and the 20-kDa myosin light chain (MLC) phosphorylation without inhibiting Ca(2+) mobilization in rabbit aortic VSM. dbcAMP mimicked these forskolin effects. We recently suggested that Ca(2+)-mediated Rho activation is dependent on class II alpha-isoform of phosphoinositide 3-kinase (PI3K-C2alpha). Forskolin inhibited KCl-induced stimulation of PI3K-C2alpha activity. KCl-induced membrane depolarization stimulated Rho in a manner dependent on a PI3K but not PKC and stimulated phosphorylation of MYPT1 at Thr(850) and CPI-17 at Thr(38) in manners dependent on both PI3K and Rho kinase, but not PKC. Forskolin, dbcAMP, and isoproterenol inhibited KCl-induced Rho activation and phosphorylation of MYPT1 and CPI-17. Consistent with these data, forskolin, isoproterenol, a PI3K inhibitor, or a Rho kinase inhibitor, but not a PKC inhibitor, abolished KCl-induced diphosphorylation of MLC. These observations indicate that cAMP inhibits Ca(2+)-mediated activation of the MLCP-regulating signaling pathway comprising PI3K-C2alpha, Rho, and Rho kinase in a manner independent of Ca(2+) and point to the novel mechanism of the cAMP actions in the regulation of vascular smooth muscle contraction. | Western Blotting | Mouse | 17110524
 |
Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase.
Fagan, KA; Oka, M; Bauer, NR; Gebb, SA; Ivy, DD; Morris, KG; McMurtry, IF
American journal of physiology. Lung cellular and molecular physiology
287
L656-64
2004
Pokaż streszczenie
RhoA GTPase mediates a variety of cellular responses, including activation of the contractile apparatus, growth, and gene expression. Acute hypoxia activates RhoA and, in turn, its downstream effector, Rho-kinase, and previous studies in rats have suggested a role for Rho/Rho-kinase signaling in both acute and chronically hypoxic pulmonary vasoconstriction. We therefore hypothesized that activation of Rho/Rho-kinase in the pulmonary circulation of mice contributes to acute hypoxic pulmonary vasoconstriction and chronic hypoxia-induced pulmonary hypertension and vascular remodeling. In isolated, salt solution-perfused mouse lungs, acute administration of the Rho-kinase inhibitor Y-27632 (1 x 10(-5) M) attenuated hypoxic vasoconstriction as well as that due to angiotensin II and KCl. Chronic treatment with Y-27632 (30 mg x kg(-1) x day(-1)) via subcutaneous osmotic pump decreased right ventricular systolic pressure, right ventricular hypertrophy, and neomuscularization of the distal pulmonary vasculature in mice exposed to hypobaric hypoxia for 14 days. Analysis of a small number of proximal pulmonary arteries suggested that Y-27632 treatment reduced the level of phospho-CPI-17, a Rho-kinase target, in hypoxic lungs. We also found that endothelial nitric oxide synthase protein in hypoxic lungs was augmented by Y-27632, suggesting that enhanced nitric oxide production might have played a role in the Y-27632-induced attenuation of chronically hypoxic pulmonary hypertension. In conclusion, Rho/Rho-kinase activation is important in the effects of both acute and chronic hypoxia on the pulmonary circulation of mice, possibly by contributing to both vasoconstriction and vascular remodeling. | Western Blotting | Mouse | 14977625
 |
Solution NMR structure of the myosin phosphatase inhibitor protein CPI-17 shows phosphorylation-induced conformational changes responsible for activation.
Ohki, S, et al.
J. Mol. Biol., 314: 839-49 (2001)
2001
Pokaż streszczenie
Contractility of vascular smooth muscle depends on phosphorylation of myosin light chains, and is modulated by hormonal control of myosin phosphatase activity. Signaling pathways activate kinases such as PKC or Rho-dependent kinases that phosphorylate the myosin phosphatase inhibitor protein called CPI-17. Phosphorylation of CPI-17 at Thr38 enhances its inhibitory potency 1000-fold, creating a molecular on/off switch for regulating contraction. We report the solution NMR structure of the CPI-17 inhibitory domain (residues 35-120), which retains the signature biological properties of the full-length protein. The final ensemble of 20 sets of NMR coordinates overlaid onto their mean structure with r.m.s.d. values of 0.84(+/-0.22) A for the backbone atoms. The protein forms a novel four-helix, V-shaped bundle comprised of a central anti-parallel helix pair (B/C helices) flanked by two large spiral loops formed by the N and C termini that are held together by another anti-parallel helix pair (A/D helices) stabilized by intercalated aromatic and aliphatic side-chains. Chemical shift perturbations indicated that phosphorylation of Thr38 induces a conformational change involving displacement of helix A, without significant movement of the other three helices. This conformational change seems to flex one arm of the molecule, thereby exposing new surfaces of the helix A and the nearby phosphorylation loop to form specific interactions with the catalytic site of the phosphatase. This phosphorylation-dependent conformational change offers new structural insights toward understanding the specificity of CPI-17 for myosin phosphatase and its function as a molecular switch. | | | 11734001
 |