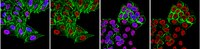
Histone deacetylase expression in white matter oligodendrocytes after stroke.
Kassis, H; Chopp, M; Liu, XS; Shehadah, A; Roberts, C; Zhang, ZG
Neurochemistry international
77
17-23
2014
Pokaż streszczenie
Histone deacetylases (HDACs) constitute a super-family of enzymes grouped into four major classes (Class I-IV) that deacetylate histone tails leading to chromatin condensation and gene repression. Whether stroke-induced oligodendrogenesis is related to the expression of individual HDACs in the oligodendrocyte lineage has not been investigated. We found that 2 days after stroke, oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes (OLGs) were substantially reduced in the peri-infarct corpus callosum, whereas at 7 days after stroke, a robust increase in OPCs and OLGs was observed. Ischemic brains isolated from rats sacrificed 7 days after stroke were used to test levels of individual members of Class I (1 and 2) and Class II (4 and 5) HDACs in white matter oligodendrocytes during stroke-induced oligodendrogenesis. Double immunohistochemistry analysis revealed that stroke substantially increased the number of NG2+OPCs with nuclear HDAC1 and HDAC2 immunoreactivity and cytoplasmic HDAC4 which were associated with augmentation of proliferating OPCs, as determined by BrdU and Ki67 double reactive cells after stroke. A decrease in HDAC1 and an increase in HDAC2 immunoreactivity were detected in mature adenomatous polyposis coli (APC) positive OLGs, which paralleled an increase in newly generated BrdU positive OLGs in the peri-infarct corpus callosum. Concurrently, stroke substantially decreased the acetylation levels of histones H3 and H4 in both OPCs and OLGs. Taken together, these findings demonstrate that stroke induces distinct profiles of Class I and Class II HDACs in white matter OPCs and OLGs, suggesting that the individual members of Class I and II HDACs play divergent roles in the regulation of OPC proliferation and differentiation during brain repair after stroke. | 24657831
 |
Essential role of DPPA3 for chromatin condensation in mouse oocytogenesis.
Liu, YJ; Nakamura, T; Nakano, T
Biology of reproduction
86
40
2011
Pokaż streszczenie
Dynamic alterations in chromatin configuration occur in mammalian oocytogenesis. Based on chromatin configuration patterns, fully grown oocytes are classified into two types. One is surrounded nucleolus (SN)-type and the other is nonsurrounded nucleolus (NSN)-type oocytes. Although chromatin condensation during the transition from NSN- to SN-type oocytes is a prerequisite for normal early embryonic development, the molecular mechanisms remain unclear. In this study, we analyzed the role of DPPA3 (also known as PGC7/Stella) in this transition using Dppa3-null oocytes. The NSN-to-SN transition was significantly impaired, and transcriptional repression was incomplete in the Dppa3-null oocytes. Additionally, we revealed that prior transcriptional repression was necessary for the NSN-to-SN transition. These findings demonstrate that DPPA3 is an essential factor for the production of functional oocytes through transcriptional repression and chromatin condensation. | 22034526
 |
Impaired coenzyme A synthesis in fission yeast causes defective mitosis, quiescence-exit failure, histone hypoacetylation and fragile DNA.
Nakamura, T; Pluskal, T; Nakaseko, Y; Yanagida, M
Open biology
2
120117
2011
Pokaż streszczenie
Biosynthesis of coenzyme A (CoA) requires a five-step process using pantothenate and cysteine in the fission yeast Schizosaccharomyces pombe. CoA contains a thiol (SH) group, which reacts with carboxylic acid to form thioesters, giving rise to acyl-activated CoAs such as acetyl-CoA. Acetyl-CoA is essential for energy metabolism and protein acetylation, and, in higher eukaryotes, for the production of neurotransmitters. We isolated a novel S. pombe temperature-sensitive strain ppc1-537 mutated in the catalytic region of phosphopantothenoylcysteine synthetase (designated Ppc1), which is essential for CoA synthesis. The mutant becomes auxotrophic to pantothenate at permissive temperature, displaying greatly decreased levels of CoA, acetyl-CoA and histone acetylation. Moreover, ppc1-537 mutant cells failed to restore proliferation from quiescence. Ppc1 is thus the product of a super-housekeeping gene. The ppc1-537 mutant showed combined synthetic lethal defects with five of six histone deacetylase mutants, whereas sir2 deletion exceptionally rescued the ppc1-537 phenotype. In synchronous cultures, ppc1-537 cells can proceed to the S phase, but lose viability during mitosis failing in sister centromere/kinetochore segregation and nuclear division. Additionally, double-strand break repair is defective in the ppc1-537 mutant, producing fragile broken DNA, probably owing to diminished histone acetylation. The CoA-supported metabolism thus controls the state of chromosome DNA. | 23091701
 |