Proteasome Inhibition Alleviates SNARE-Dependent Neurodegeneration.
Sharma, Manu, et al.
Sci Transl Med, 4: 147ra113 (2012)
2011
Pokaż streszczenie
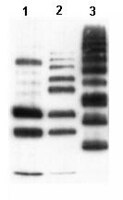
Activation of the proteasomal degradation of misfolded proteins has been proposed as a therapeutic strategy for treating neurodegenerative diseases, but it is unclear whether proteasome dysfunction contributes to neurodegeneration. We tested the role of proteasome activity in neurodegeneration developed by mice lacking cysteine string protein-α (CSPα). Unexpectedly, we found that proteasome inhibitors alleviated neurodegeneration in CSPα-deficient mice, reversing impairment of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-complex assembly and extending life span. We tested whether dysfunctional SNARE-complex assembly could contribute to neurodegeneration in Alzheimer's and Parkinson's disease by analyzing postmortem brain tissue from these patients; we found reduced SNARE-complex assembly in the brain tissue samples. Our results suggest that proteasomal activation may not always be beneficial for alleviating neurodegeneration and that blocking the proteasome may represent a potential therapeutic avenue for treating some forms of neurodegenerative disease. | 22896677
 |
z-Leucinyl-leucinyl-norleucinal induces apoptosis of human glioblastoma tumor-initiating cells by proteasome inhibition and mitotic arrest response.
Monticone M, Biollo E, Fabiano A, Fabbi M, Daga A, Romeo F, Maffei M, Melotti A, Giaretti W, Corte G, Castagnola P
Molecular cancer research : MCR
7
1822-34
2009
Pokaż streszczenie
Gamma-secretase inhibitors have been proposed as drugs able to kill cancer cells by targeting the NOTCH pathway. Here, we investigated two of such inhibitors, the Benzyloxicarbonyl-Leu-Leu-Nle-CHO (LLNle) and the N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), to assess whether they were effective in killing human glioblastoma tumor-initiating cells (GBM TIC) in vitro. We found that only LLNle was able at the micromolar range to induce the death of GBM TICs by apoptosis. To determine the cellular processes that were activated in GBM TICs by treatment with LLNle, we analyzed the amount of the NOTCH intracellular domain and the gene expression profiles following treatment with LLNle, DAPT, and DMSO (vehicle). We found that LLNIe, beside inhibiting the generation of the NOTCH intracellular domain, also induces proteasome inhibition, proteolytic stress, and mitotic arrest in these cells by repressing genes required for DNA synthesis and mitotic progression and by activating genes acting as mitotic inhibitors. DNA content flow cytometry clearly showed that cells treated with LLNle undergo arrest in the G(2)-M phases of the cell cycle. We also found that DAPT and L-685,458, another selective Notch inhibitor, were unable to kill GBM TICs, whereas lactacystin, a pure proteasome inhibitor, was effective although at a much less extent than LLNle. These data show that LLNle kills GBM TIC cells by inhibiting the proteasome activity. We suggest that LLNle, being able to target two relevant pathways for GBM TIC survival,-05-have a potential therapeutic value that deserves further investigation in animal models. | 19861404
 |
PI31 is a modulator of proteasome formation and antigen processing.
Zaiss, Dietmar M W, et al.
Proc. Natl. Acad. Sci. U.S.A., 99: 14344-9 (2002)
2002
Pokaż streszczenie
Regulation of the proteasome system, which is responsible for the generation of most MHC class I-bound peptides, occurs through the interaction of the 20S proteasome with several regulatory proteins. One of these is PI31, which acts in vitro as an inhibitor of proteasome activity. Here, we demonstrate that, rather than inhibiting proteasome function, PI31 acts as a selective modulator of the proteasome-mediated steps in MHC class I antigen processing. Overexpression of PI31 in mouse embryonic cells has no impact on proteasome-mediated proteolysis. Instead, PI31, which localizes at the nuclear envelope/endoplasmic reticulum membrane, selectively interferes with the maturation of immunoproteasome precursor complexes. Consequently, overexpression of PI31 abrogates MHC class I presentation of an immunoproteasome-dependent cytotoxic T lymphocyte epitope and reduces the surface MHC class I levels on IFN-gamma-treated mouse embryonic cells. Thus, PI31 represents a cellular regulator of proteasome formation and of proteasome-mediated antigen processing. | 12374861
 |
Transient aggregation of ubiquitinated proteins during dendritic cell maturation.
Lelouard, Hugues, et al.
Nature, 417: 177-82 (2002)
2002
Pokaż streszczenie
Dendritic cells (DCs) are antigen-presenting cells with the unique capacity to initiate primary immune responses. Dendritic cells have a remarkable pattern of differentiation (maturation) that exhibits highly specific mechanisms to control antigen presentation restricted by major histocompatibility complex (MHC). MHC class I molecules present to CD8(+) cytotoxic T cells peptides that are derived mostly from cytosolic proteins, which are ubiquitinated and then degraded by the proteasome. Here we show that on inflammatory stimulation, DCs accumulate newly synthesized ubiquitinated proteins in large cytosolic structures. These structures are similar to, but distinct from, aggresomes and inclusion bodies observed in many amyloid diseases. Notably, these dendritic cell aggresome-like induced structures (DALIS) are transient, require continuous protein synthesis and do not affect the ubiquitin-proteasome pathway. Our observations suggest the existence of an organized prioritization of protein degradation in stimulated DCs, which is probably important for regulating MHC class I presentation during maturation. | 12000969
 |