TCF4 Is a Molecular Target of Resveratrol in the Prevention of Colorectal Cancer.
Jeong, JB; Lee, J; Lee, SH
International journal of molecular sciences
16
10411-25
2015
Pokaż streszczenie
The Wnt/β-catenin pathway plays an essential role in the tumorigenesis of colorectal cancer. T-cell factor-4 (TCF4) is a member of the TCF/LEF (lymphoid enhancer factor) family of transcription factors, and dysregulation of β-catenin is decisive for the initiation and progression of colorectal cancer. However, the role of TCF4 in the transcriptional regulation of its target gene remained poorly understood. Resveratrol is a dietary phytoalexin and present in many plants, including grape skin, nuts and fruits. Although resveratrol has been widely implicated in anti-tumorigenic and pro-apoptotic properties in several cancer models, the underlying cellular mechanisms are only partially understood. The current study was performed to elucidate the molecular mechanism of the anti-cancer activity of resveratrol in human colorectal cancer cells. The treatment of resveratrol and other phytochemicals decreased the expression of TCF4. Resveratrol decreases cellular accumulation of exogenously-introduced TCF4 protein, but did not change the TCF4 transcription. The inhibition of proteasomal degradation using MG132 (carbobenzoxy-Leu-Leu-leucinal) and lactacystin ameliorates resveratrol-stimulated down-regulation of TCF4. The half-life of TCF4 was decreased in the cells exposed to resveratrol. Resveratrol increased phosphorylation of TCF4 at serine/threonine residues through ERK (extracellular signal-regulated kinases) and p38-dependent pathways. The TCF4 knockdown decreased TCF/β-catenin-mediated transcriptional activity and sensitized resveratrol-induced apoptosis. The current study provides a new mechanistic link between resveratrol and TCF4 down-regulation and significant benefits for further preclinical and clinical practice. | | 25961950
 |
Two novel type 2 diabetes loci revealed through integration of TCF7L2 DNA occupancy and SNP association data.
Johnson, ME; Zhao, J; Schug, J; Deliard, S; Xia, Q; Guy, VC; Sainz, J; Kaestner, KH; Wells, AD; Grant, SF
BMJ Open Diabetes Res Care
2
e000052
2014
Pokaż streszczenie
The transcription factor 7-like 2 (TCF7L2) locus is strongly implicated in the pathogenesis of type 2 diabetes (T2D). We previously mapped the genomic regions bound by TCF7L2 using ChIP (chromatin immunoprecipitation)-seq in the colorectal carcinoma cell line, HCT116, revealing an unexpected highly significant over-representation of genome-wide association studies (GWAS) loci associated primarily with endocrine (in particular T2D) and cardiovascular traits.In order to further explore if this observed phenomenon occurs in other cell lines, we carried out ChIP-seq in HepG2 cells and leveraged ENCODE data for five additional cell lines. Given that only a minority of the predicted genetic component to most complex traits has been identified to date, plus our GWAS-related observations with respect to TCF7L2 occupancy, we investigated if restricting association analyses to the genes yielded from this approach, in order to reduce the constraints of multiple testing, could reveal novel T2D loci.We found strong evidence for the continued enrichment of endocrine and cardiovascular GWAS categories, with additional support for cancer. When investigating all the known GWAS loci bound by TCF7L2 in the shortest gene list, derived from HCT116, the coronary artery disease-associated variant, rs46522 at the UBE2Z-GIP-ATP5G1-SNF8 locus, yielded significant association with T2D within DIAGRAM. Furthermore, when we analyzed tag-SNPs (single nucleotide polymorphisms) in genes not previously implicated by GWAS but bound by TCF7L2 within 5 kb, we observed a significant association of rs4780476 within CPPED1 in DIAGRAM.ChIP-seq data generated with this GWAS-implicated transcription factor provided a biologically plausible method to limit multiple testing in the assessment of genome-wide genotyping data to uncover two novel T2D-associated loci. | | 25469308
 |
Parathyroid hormone-related protein inhibits DKK1 expression through c-Jun-mediated inhibition of β-catenin activation of the DKK1 promoter in prostate cancer.
Zhang, H; Yu, C; Dai, J; Keller, JM; Hua, A; Sottnik, JL; Shelley, G; Hall, CL; Park, SI; Yao, Z; Zhang, J; McCauley, LK; Keller, ET
Oncogene
33
2464-77
2014
Pokaż streszczenie
Prostate cancer (PCa)bone metastases are unique in that majority of them induce excessive mineralized bone matrix, through undefined mechanisms, as opposed to most other cancers that induce bone resorption. Parathyroid hormone-related protein (PTHrP) is produced by PCa cells and intermittent PTHrP exposure has bone anabolic effects, suggesting that PTHrP could contribute to the excess bone mineralization. Wnts are bone-productive factors produced by PCa cells, and the Wnt inhibitor Dickkopfs-1 (DKK1) has been shown to promote PCa progression. These findings, in conjunction with the observation that PTHrP expression increases and DKK1 expression decreases as PCa progresses, led to the hypothesis that PTHrP could be a negative regulator of DKK1 expression in PCa cells and, hence, allow the osteoblastic activity of Wnts to be realized. To test this, we first demonstrated that PTHrP downregulated DKK1 mRNA and protein expression. We then found through multiple mutated DKK1 promoter assays that PTHrP, through c-Jun activation, downregulated the DKK1 promoter through a transcription factor (TCF) response element site. Furthermore, chromatin immunoprecipitation (ChIP) and re-ChIP assays revealed that PTHrP mediated this effect through inducing c-Jun to bind to a transcriptional activator complex consisting of β-catenin, which binds the most proximal DKK1 promoter, the TCF response element. Together, these results demonstrate a novel signaling linkage between PTHrP and Wnt signaling pathways that results in downregulation of a Wnt inhibitor allowing for Wnt activity that could contribute the osteoblastic nature of PCa. | Chromatin Immunoprecipitation (ChIP) | 23752183
 |
Nuclear AXIN2 represses MYC gene expression.
Rennoll, SA; Konsavage, WM; Yochum, GS
Biochemical and biophysical research communications
443
217-22
2014
Pokaż streszczenie
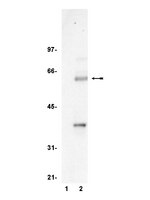
The β-catenin transcriptional coactivator is the key mediator of the canonical Wnt signaling pathway. In the absence of Wnt, β-catenin associates with a cytosolic and multi-protein destruction complex where it is phosphorylated and targeted for proteasomal degradation. In the presence of Wnt, the destruction complex is inactivated and β-catenin translocates into the nucleus. In the nucleus, β-catenin binds T-cell factor (TCF) transcription factors to activate expression of c-MYC (MYC) and Axis inhibition protein 2 (AXIN2). AXIN2 is a member of the destruction complex and, thus, serves in a negative feedback loop to control Wnt/β-catenin signaling. AXIN2 is also present in the nucleus, but its function within this compartment is unknown. Here, we demonstrate that AXIN2 localizes to the nuclei of epithelial cells within normal and colonic tumor tissues as well as colorectal cancer cell lines. In the nucleus, AXIN2 represses expression of Wnt/β-catenin-responsive luciferase reporters and forms a complex with β-catenin and TCF. We demonstrate that AXIN2 co-occupies β-catenin/TCF complexes at the MYC promoter region. When constitutively localized to the nucleus, AXIN2 alters the chromatin structure at the MYC promoter and directly represses MYC gene expression. These findings suggest that nuclear AXIN2 functions as a rheostat to control MYC expression in response to Wnt/β-catenin signaling. | Western Blotting | 24299953
 |
The myc 3' wnt-responsive element suppresses colonic tumorigenesis.
Konsavage, WM; Yochum, GS
Molecular and cellular biology
34
1659-69
2014
Pokaż streszczenie
Mutations in components of the Wnt/β-catenin signaling pathway are commonly found in colorectal cancers, and these mutations cause aberrant expression of genes controlled by Wnt-responsive DNA elements (WREs). While the c-Myc proto-oncogene (Myc) is required for intestinal phenotypes associated with pathogenic Wnt/β-catenin signaling in vivo, the WREs that control Myc expression in this setting have yet to be fully described. Previously, we demonstrated that the Myc 3' WRE was required for intestinal homeostasis and intestinal repair in response to damage. Here, we tested the role of the Myc 3' WRE in intestinal tumorigenesis using two independent mouse models. In comparison to Apc(Min/+) mice, Apc(Min/+) Myc 3' WRE(-/-) mice contained 25% fewer tumors in the small intestine. Deletion of the Myc 3' WRE(-/-) in the Apc(Min/+) background resulted in 4-fold more colonic tumors. In a model of colitis-associated colorectal cancer, the Myc 3' WRE suppressed colonic tumorigenesis, most notably within the cecum. Using chromatin immunoprecipitation and transcript analysis of purified colonic crypts, we found that the Myc 3' WRE is required for the transcriptional regulation of Myc expression in vivo. These results emphasize the critical role of the Myc 3' WRE in maintaining homeostatic Myc expression. | | 24567369
 |
Genome-wide ChIP-seq analysis of TCF4 binding regions in colorectal cancer cells.
Chen, C; Lu, Y; Liu, J; Li, L; Zhao, N; Lin, B
International journal of clinical and experimental medicine
7
4253-9
2014
Pokaż streszczenie
TCF4 (transcriptional factor 4) forms a complex with its transcriptional coactivator β-catenin and the coactivator carries the final signal output from the canonical Wnt signaling pathway, which is essential for the growth of normal epithelium and also plays important roles in carcinogenesis of colon epithelium. We aimed to gain a better understanding of the genes bound by TCF4 in colorectal cancer cells.SW620 human colorectal cancer cells were cultured. The TCF4 antibody of this study was confirmed in SW620 cells by Western Blot. A ChIP-seq based genome-wide analysis of TCF4 chromatin occupancy in colorectal cancer cells was conducted and 1506 high confidence TCF4 binding sites wereidentified.Sequence analysis revealed that the binding sites harbor a consensus sequence of C-G/C-A-G-C-T/C-C-T-T-C. Gene ontology and pathway analysis showed that TCF4 regulated 18 genes in Wnt signaling pathway and 97 other transcription factors.Our results suggest TCF4 binding regions were enriched with a motif of C-G/C-A-G-C-T/C-C-T-T-C. The gene regulation of TCF4 may be conserved in colorectal cancer and glioma cells. TCF4 may be involved in a series of important biological processes such as regulation of metabolic and biosynthetic (GO: 0010604, GO: 0031328, GO: 0009891, GO: 0051173, GO: 0010557, GO: 0045935), adhesion (GO: 0007155, GO: 0022610), apoptosis (GO: 0042981, GO: 0043067, GO: 0010941), and important signaling pathways (Wnt, Chemokine, Calciu, GnRH). | | 25550940
 |
HIV's Nef interacts with β-catenin of the Wnt signaling pathway in HEK293 cells.
Weiser, K; Barton, M; Gershoony, D; Dasgupta, R; Cardozo, T
PloS one
8
e77865
2013
Pokaż streszczenie
The Wnt signaling pathway is implicated in major physiologic cellular functions, such as proliferation, migration, cell fate specification, maintenance of pluripotency and induction of tumorigenicity. Proliferation and migration are important responses of T-cells, which are major cellular targets of HIV infection. Using an informatics screen, we identified a previously unsuspected interaction between HIV's Nef protein and β-catenin, a key component of the Wnt pathway. A segment in Nef contains identical amino acids at key positions and structurally mimics the β-catenin binding sites on endogenous β-catenin ligands. The interaction between Nef and β-catenin was confirmed in vitro and in a co-immunoprecipitation from HEK293 cells. Moreover, the introduction of Nef into HEK293 cells specifically inhibited a Wnt pathway reporter. | Western Blotting | 24130899
 |
Human SMC2 protein, a core subunit of human condensin complex, is a novel transcriptional target of the WNT signaling pathway and a new therapeutic target.
Dávalos, V; Súarez-López, L; Castaño, J; Messent, A; Abasolo, I; Fernandez, Y; Guerra-Moreno, A; Espín, E; Armengol, M; Musulen, E; Ariza, A; Sayós, J; Arango, D; Schwartz, S
The Journal of biological chemistry
287
43472-81
2011
Pokaż streszczenie
Human SMC2 is part of the condensin complex, which is responsible for tightly packaging replicated genomic DNA prior to segregation into daughter cells. Engagement of the WNT signaling pathway is known to have a mitogenic effect on cells, but relatively little is known about WNT interaction with mitotic structural organizer proteins. In this work, we described the novel transcriptional regulation of SMC2 protein by direct binding of the β-catenin·TCF4 transcription factor to the SMC2 promoter. Furthermore, we identified the precise region in the SMC2 promoter that is required for β-catenin-mediated promoter activation. Finally, we explored the functional significance of down-regulating SMC2 protein in vivo. Treatment of WNT-activated intestinal tumor cells with SMC2 siRNA significantly reduced cell proliferation in nude mice, compared with untreated controls (p = 0.02). Therefore, we propose that WNT signaling can directly activate SMC2 transcription as a key player in the mitotic cell division machinery. Furthermore, SMC2 represents a new target for oncological therapeutic intervention. | Western Blotting | 23095742
 |
Regulation of amphiregulin gene expression by β-catenin signaling in human hepatocellular carcinoma cells: a novel crosstalk between FGF19 and the EGFR system.
Latasa, MU; Salis, F; Urtasun, R; Garcia-Irigoyen, O; Elizalde, M; Uriarte, I; Santamaria, M; Feo, F; Pascale, RM; Prieto, J; Berasain, C; Avila, MA
PloS one
7
e52711
2011
Pokaż streszczenie
Hepatocellular carcinoma (HCC) is the most prevalent liver tumor and a deadly disease with limited therapeutic options. Dysregulation of cell signaling pathways is a common denominator in tumorigenesis, including hepatocarcinogenesis. The epidermal growth factor receptor (EGFR) signaling system is commonly activated in HCC, and is currently being evaluated as a therapeutic target in combination therapies. We and others have identified a central role for the EGFR ligand amphiregulin (AR) in the proliferation, survival and drug resistance of HCC cells. AR expression is frequently up-regulated in HCC tissues and cells through mechanisms not completely known. Here we identify the β-catenin signaling pathway as a novel mechanism leading to transcriptional activation of the AR gene in human HCC cells. Activation of β-catenin signaling, or expression of the T41A β-catenin active mutant, led to the induction of AR expression involving three specific β-catenin-Tcf responsive elements in its proximal promoter. We demonstrate that HCC cells expressing the T41A β-catenin active mutant show enhanced proliferation that is dependent in part on AR expression and EGFR signaling. We also demonstrate here a novel cross-talk of the EGFR system with fibroblast growth factor 19 (FGF19). FGF19 is a recently identified driver gene in hepatocarcinogenesis and an activator of β-catenin signaling in HCC and colon cancer cells. We show that FGF19 induced AR gene expression through the β-catenin pathway in human HCC cells. Importantly, AR up-regulation and EGFR signaling participated in the induction of cyclin D1 and cell proliferation elicited by FGF19. Finally, we demonstrate a positive correlation between FGF19 and AR expression in human HCC tissues, therefore supporting in clinical samples our experimental observations. These findings identify the AR/EGFR system as a key mediator of FGF19 responses in HCC cells involving β-catenin signaling, and suggest that combined targeting of FGF19 and AR/EGFR may enhance therapeutic efficacy. | | 23285165
 |
Review of the neuroanatomic landscape implicated in glucose sensing and regulation of nutrient signaling: immunophenotypic localization of diabetes gene Tcf7l2 in the developing murine brain.
Weaver, C; Turner, N; Hall, J
Journal of chemical neuroanatomy
45
1-17
2011
Pokaż streszczenie
Genetic variants in the transcription factor 7-like 2(Tcf7l2) gene have been found to confer a significant risk of type 2 diabetes and attenuated insulin secretion. Based on its genomic wide association Tcf7l2 is considered the single most important predictor of diabetes to date. Previous studies of Tcf7l2 mRNA localization in the adult brain suggest a putative role of Tcf7l2 in the CNS regulation of energy homeostasis. The present study further characterizes the immunophenotypic distribution of peptide expression in the brains of Tcf7l2 progeny during developmental time periods between E12.5 and P1. Tcf7l2(-/-) is lethal beyond P1. Results show that while negligible TCF7L2 expression is found in the developing brains of Tcf7l2(-/-)mice, TCF7L2 protein is relatively widespread and robustly expressed in the brain by E18.5 and exhibits specific expression within neuronal populations and regions of the brain in Tcf7l2(+/-) and Tcf7l2(+/+) progeny. Strong immunophenotypic labeling was found in the diencephalic structure of the thalamus that suggests a role of Tcf7l2 in the development and maintenance of thalamic activity. Strongly expressed TCF7L2 was localized in select hypothalamic and preoptic nuclei indicative of Tcf7l2 function within neurons controlling energy balance. Definitive neuronal staining for TCF7L2 within nuclei of the brain stem and circumventricular organs extends TCF7L2 localization within autonomic neurons and its potential integration with autonomic function. In addition robust TCF7L2 expression was found in the tectal and tegmental structures of the superior and inferior colliculi as well as transient expression in neuroepithelium of the cerebral and hippocampal cortices of E16 and E18.5. Patterns of TCF7L2 peptide localization when compared to the adult protein synthetic chemical/anatomical landscape of glucose sensing exhibit a good correlational fit between its expression and regions, nuclei, and pathways regulating energy homeostasis via integration and response to peripheral endocrine, metabolic and neuronal signaling. TCF was also found co-localized with peptides that regulate energy homeostasis including AgRP, POMC and NPY. TCF7l2, some variants of which have been shown to impair GLP-1-induced insulin secretion, was also found co-localize with GLP-1 in adult TCF wild type progeny. Impaired Tcf7l2-mediated neural regulation may contribute to the risk and/or underlying pathophysiology of type 2 diabetes that has found high expression in genomic studies of Tcf7l2 variants. | Immunohistochemistry | 22796301
 |