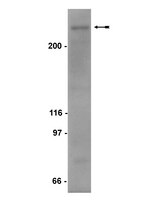
NCoR1 and SMRT play unique roles in thyroid hormone action in vivo.
Shimizu, H; Astapova, I; Ye, F; Bilban, M; Cohen, RN; Hollenberg, AN
Molecular and cellular biology
35
555-65
2015
Pokaż streszczenie
NCoR1 (nuclear receptor corepressor) and SMRT (silencing mediator of retinoid and thyroid hormone receptors; NCoR2) are well-recognized coregulators of nuclear receptor (NR) action. However, their unique roles in the regulation of thyroid hormone (TH) signaling in specific cell types have not been determined. To accomplish this we generated mice that lacked function of either NCoR1, SMRT, or both in the liver only and additionally a global SMRT knockout model. Despite both corepressors being present in the liver, deletion of SMRT in either euthyroid or hypothyroid animals had little effect on TH signaling. In contrast, disruption of NCoR1 action confirmed that NCoR1 is the principal mediator of TH sensitivity in vivo. Similarly, global disruption of SMRT, unlike the global disruption of NCoR1, did not affect TH levels. While SMRT played little role in TH-regulated pathways, when disrupted in combination with NCoR1, it greatly accentuated the synthesis and storage of hepatic lipid. Taken together, these data demonstrate that corepressor specificity exists in vivo and that NCoR1 is the principal regulator of TH action. However, both corepressors collaborate to control hepatic lipid content, which likely reflects their cooperative activity in regulating the action of multiple NRs including the TH receptor (TR). | | 25421714
 |
Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes.
Caron, S; Huaman Samanez, C; Dehondt, H; Ploton, M; Briand, O; Lien, F; Dorchies, E; Dumont, J; Postic, C; Cariou, B; Lefebvre, P; Staels, B
Molecular and cellular biology
33
2202-11
2013
Pokaż streszczenie
The glucose-activated transcription factor carbohydrate response element binding protein (ChREBP) induces the expression of hepatic glycolytic and lipogenic genes. The farnesoid X receptor (FXR) is a nuclear bile acid receptor controlling bile acid, lipid, and glucose homeostasis. FXR negatively regulates hepatic glycolysis and lipogenesis in mouse liver. The aim of this study was to determine whether FXR regulates the transcriptional activity of ChREBP in human hepatocytes and to unravel the underlying molecular mechanisms. Agonist-activated FXR inhibits glucose-induced transcription of several glycolytic genes, including the liver-type pyruvate kinase gene (L-PK), in the immortalized human hepatocyte (IHH) and HepaRG cell lines. This inhibition requires the L4L3 region of the L-PK promoter, known to bind the transcription factors ChREBP and hepatocyte nuclear factor 4α (HNF4α). FXR interacts directly with ChREBP and HNF4α proteins. Analysis of the protein complex bound to the L4L3 region reveals the presence of ChREBP, HNF4α, FXR, and the transcriptional coactivators p300 and CBP at high glucose concentrations. FXR activation does not affect either FXR or HNF4α binding to the L4L3 region but does result in the concomitant release of ChREBP, p300, and CBP and in the recruitment of the transcriptional corepressor SMRT. Thus, FXR transrepresses the expression of genes involved in glycolysis in human hepatocytes. | Western Blotting | 23530060
 |
Nuclear corepressors mediate the repression of phospholipase A2 group IIa gene transcription by thyroid hormone.
Sharma, P; Thakran, S; Deng, X; Elam, MB; Park, EA
The Journal of biological chemistry
288
16321-33
2013
Pokaż streszczenie
Secretory phospholipase A2 group IIa (PLA2g2a) is associated with inflammation, hyperlipidemia, and atherogenesis. Transcription of the PLA2g2a gene is induced by multiple cytokines. Here, we report the surprising observation that thyroid hormone (T3) inhibited PLA2g2a gene expression in human and rat hepatocytes as well as in rat liver. Moreover, T3 reduced the cytokine-mediated induction of PLA2g2a, suggesting that the thyroid status may modulate aspects of the inflammatory response. In an effort to dissect the mechanism of repression by T3, we cloned the PLA2g2a gene and identified a negative T3 response element in the promoter. This T3 receptor (TRβ)-binding site differed considerably from consensus T3 stimulatory elements. Using in vitro and in vivo binding assays, we found that TRβ bound directly to the PLA2g2a promoter as a heterodimer with the retinoid X receptor. Knockdown of nuclear corepressor or silencing mediator for retinoid and thyroid receptors by siRNA blocked the T3 inhibition of PLA2g2a. Using chromatin immunoprecipitation assays, we showed that nuclear corepressor and silencing mediator for retinoid and thyroid receptors were associated with the PLA2g2a gene in the presence of T3. In contrast with the established role of T3 to promote coactivator association with TRβ, our experiments demonstrate a novel inverse recruitment mechanism in which liganded TRβ recruits corepressors to inhibit PLA2g2a expression. | Western Blotting | 23629656
 |
Rescue of a primary myelofibrosis model by retinoid-antagonist therapy.
Hong, SH; Dvorak-Ewell, M; Stevens, HY; Barish, GD; Castro, GL; Nofsinger, R; Frangos, JA; Shoback, D; Evans, RM
Proceedings of the National Academy of Sciences of the United States of America
110
18820-5
2013
Pokaż streszczenie
Molecular targeting of the two receptor interaction domains of the epigenetic repressor silencing mediator of retinoid and thyroid hormone receptors (SMRT(mRID)) produced a transplantable skeletal syndrome that reduced radial bone growth, increased numbers of bone-resorbing periosteal osteoclasts, and increased bone fracture risk. Furthermore, SMRT(mRID) mice develop spontaneous primary myelofibrosis, a chronic, usually idiopathic disorder characterized by progressive bone marrow fibrosis. Frequently linked to polycythemia vera and chronic myeloid leukemia, myelofibrosis displays high patient morbidity and mortality, and current treatment is mostly palliative. To decipher the etiology of this disease, we identified the thrombopoietin (Tpo) gene as a target of the SMRT-retinoic acid receptor signaling pathway in bone marrow stromal cells. Chronic induction of Tpo in SMRT(mRID) mice results in up-regulation of TGF-β and PDGF in megakaryocytes, uncontrolled proliferation of bone marrow reticular cells, and fibrosis of the marrow compartment. Of therapeutic relevance, we show that this syndrome can be rescued by retinoid antagonists, demonstrating that the physical interface between SMRT and retinoic acid receptor can be a potential therapeutic target to block primary myelofibrosis disease progression. | | 24191050
 |
The Stat3/GR Interaction Code: Predictive Value of Direct/Indirect DNA Recruitment for Transcription Outcome.
David Langlais,Catherine Couture,Aurélio Balsalobre,Jacques Drouin
Molecular cell
47
2011
Pokaż streszczenie
Transcription factor recruitment to genomic sites of action is primarily due to direct protein:DNA interactions. The subsequent recruitment of coregulatory complexes leads to either transcriptional activation or repression. In contrast to this canonical scheme, some transcription factors, such as the glucocorticoid receptor (GR), behave as transcriptional repressors when recruited to target genes through protein tethering. We have investigated the genome-wide prevalence of tethering between GR and Stat3 and found nonreciprocal interactions, namely that GR tethering to DNA-bound Stat3 results in transcriptional repression, whereas Stat3 tethering to GR results in synergism. Further, other schemes of GR and Stat3 corecruitment to regulatory modules result in transcriptional synergism, including neighboring and composite binding sites. The results indicate extensive transcriptional interactions between Stat3 and GR; further, they provide a genome-wide assessment of transcriptional regulation by tethering and a molecular basis for integration of signals mediated by GR and Stats in health and disease. | | 22633955
 |
TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells.
Takahashi, H; Kanno, T; Nakayamada, S; Hirahara, K; Sciumè, G; Muljo, SA; Kuchen, S; Casellas, R; Wei, L; Kanno, Y; O'Shea, JJ
Nature immunology
13
587-95
2011
Pokaż streszczenie
Distinct CD4(+) T cell subsets are critical for host defense and immunoregulation. Although these subsets can act as terminally differentiated lineages, they have been increasingly noted to demonstrated plasticity. MicroRNAs are factors that control T cell stability and plasticity. Here we report that naturally occurring regulatory T cells (T(reg) cells) had high expression of the microRNA miR-10a and that miR-10a was induced by retinoic acid and transforming growth factor-β (TGF-β) in inducible T(reg) cells. By simultaneously targeting the transcriptional repressor Bcl-6 and the corepressor Ncor2, miR-10a attenuated the phenotypic conversion of inducible T(reg) cells into follicular helper T cells. We also found that miR-10a limited differentiation into the T(H)17 subset of helper T cells and therefore represents a factor that can fine-tune the plasticity and fate of helper T cells. | | 22544395
 |
Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation.
Blackford, JA; Guo, C; Zhu, R; Dougherty, EJ; Chow, CC; Simons, SS
The Journal of biological chemistry
287
40982-95
2011
Pokaż streszczenie
A currently obscure area of steroid hormone action is where the component factors, including receptor and reporter gene, act. The DNA binding of factors can be precisely defined, but the location and timing of factor binding and action are usually not equivalent. These questions are addressed for several factors (e.g. glucocorticoid receptor (GR), reporter, TIF2, NCoR, NELF-A, sSMRT, and STAMP) using our recently developed competition assay. This assay reveals both the kinetically defined mechanism of factor action and where the above factors act relative to both each other and the equilibrium equivalent to the rate-limiting step, which we call the concentration limiting step (CLS). The utility of this competition assay would be greatly increased if the position of the CLS is invariant and if the factor acting at the CLS is known. Here we report that the exogenous GREtkLUC reporter acts at the CLS as an accelerator for gene induction by GRs in U2OS cells. This mechanism of reporter function at the CLS persists with different reporters, factors, receptors, and cell types. We, therefore, propose that the reporter gene always acts at the CLS during gene induction and constitutes a landmark around which one can order the actions of all other factors. Current data suggest that how and where GR and the short form of SMRT act is also constant. These results validate a novel and rational methodology for identifying distally acting factors that would be attractive targets for pharmaceutical intervention in the treatment of diseases involving GR-regulated genes. | Western Blotting | 23055525
 |
GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response.
Venteclef, N; Jakobsson, T; Ehrlund, A; Damdimopoulos, A; Mikkonen, L; Ellis, E; Nilsson, LM; Parini, P; Jänne, OA; Gustafsson, JA; Steffensen, KR; Treuter, E
Genes & development
24
381-95
2009
Pokaż streszczenie
The orphan receptor LRH-1 and the oxysterol receptors LXRalpha and LXRbeta are established transcriptional regulators of lipid metabolism that appear to control inflammatory processes. Here, we investigate the anti-inflammatory actions of these nuclear receptors in the hepatic acute phase response (APR). We report that selective synthetic agonists induce SUMOylation-dependent recruitment of either LRH-1 or LXR to hepatic APR promoters and prevent the clearance of the N-CoR corepressor complex upon cytokine stimulation. Investigations of the APR in vivo, using LXR knockout mice, indicate that the anti-inflammatory actions of LXR agonists are triggered selectively by the LXRbeta subtype. We further find that hepatic APR responses in small ubiquitin-like modifier-1 (SUMO-1) knockout mice are increased, which is due in part to diminished LRH-1 action at APR promoters. Finally, we provide evidence that the metabolically important coregulator GPS2 functions as a hitherto unrecognized transrepression mediator of interactions between SUMOylated nuclear receptors and the N-CoR corepressor complex. Our study extends the knowledge of anti-inflammatory mechanisms and pathways directed by metabolic nuclear receptor-corepressor networks to the control of the hepatic APR, and implies alternative pharmacological strategies for the treatment of human metabolic diseases associated with inflammation. | | 20159957
 |
Pharmacological manipulation of the RAR/RXR signaling pathway maintains the repopulating capacity of hematopoietic stem cells in culture.
Safi, R; Muramoto, GG; Salter, AB; Meadows, S; Himburg, H; Russell, L; Daher, P; Doan, P; Leibowitz, MD; Chao, NJ; McDonnell, DP; Chute, JP
Molecular endocrinology (Baltimore, Md.)
23
188-201
2009
Pokaż streszczenie
The retinoid X receptor (RXR) contributes to the regulation of diverse biological pathways via its role as a heterodimeric partner of several nuclear receptors. However, RXR has no established role in the regulation of hematopoietic stem cell (HSC) fate. In this study, we sought to determine whether direct modulation of RXR signaling could impact human HSC self-renewal or differentiation. Treatment of human CD34(+)CD38(-)lin(-) cells with LG1506, a selective RXR modulator, inhibited the differentiation of HSCs in culture and maintained long-term repopulating HSCs in culture that were otherwise lost in response to cytokine treatment. Further studies revealed that LG1506 had a distinct mechanism of action in that it facilitated the recruitment of corepressors to the retinoic acid receptor (RAR)/RXR complex at target gene promoters, suggesting that this molecule was functioning as an inverse agonist in the context of this heterodimer. Interestingly, using combinatorial peptide phage display, we identified unique surfaces presented on RXR when occupied by LG1506 and demonstrated that other modulators that exhibited these properties functioned similarly at both a mechanistic and biological level. These data indicate that the RAR/RXR heterodimer is a critical regulator of human HSC differentiation, and pharmacological modulation of RXR signaling prevents the loss of human HSCs that otherwise occurs in short-term culture. | | 19106195
 |
Progressive loss of estrogen receptor alpha cofactor recruitment in endocrine resistance.
Naughton, C; MacLeod, K; Kuske, B; Clarke, R; Cameron, DA; Langdon, SP
Molecular endocrinology (Baltimore, Md.)
21
2615-26
2007
Pokaż streszczenie
Differential expression of estrogen receptor-alpha (ERalpha) cofactors has been implicated in endocrine resistance in breast cancer. Using a three-stage MCF-7 cell-based model that emulates the clinical manifestation of acquired endocrine resistant breast cancer we now show, using a combination of chromatin immunoprecipitation and RNA interference, that there is a progressive loss of ERalpha cofactor recruitment to the estrogen-dependent pS2 gene and reduced requirement for cofactor expression. Maximal estrogen induced pS2 induction requires ERalpha and cofactor recruitment in MCF-7 cells, but in the progression to endocrine resistance these requirements are altered and expression has become less dependent on cofactors. Additionally, in estrogen-resistant MCF-7 cells there is a global loss of requirement of individual cofactors for proliferative cell growth indicating that other genes have lost the need for transcriptional cofactors. This loss of the requirement for cofactors may represent an important mechanism for gene misregulation in cancer. | Western Blotting | 17666584
 |