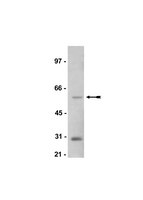
Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation.
Schweitzer, GG; Arias, EB; Cartee, GD
Journal of applied physiology (Bethesda, Md. : 1985)
113
1852-61
2011
Pokaż streszczenie
Prior exercise by rats can induce a sustained increase in muscle Akt substrate of 160 kDa (AS160) phosphorylation on Thr(642) (pAS160(Thr642)). Because phosphorylation of AS160 on both AS160(Thr642) and AS160(Ser588) is important for insulin-stimulated glucose transport (GT), we determined if exercise would also induce a sustained increase in pAS160(Ser588) concomitant with persistently elevated pAS160(Thr642) and GT. Given that the mechanisms for sustained postexercise (PEX) effects on pAS160 were uncertain, we also studied the four kinases known to phosphorylate AS160 (Akt, AMPK, RSK, and SGK1). In addition, because the serine/threonine phosphatase(s) that dephosphorylate muscle AS160 were previously unidentified, we assessed the ability of four serine/threonine phosphatases (PP1, PP2A, PP2B, and PP2C) to dephosphorylate AS160. We also evaluated exercise effects on posttranslational modifications (Tyr(307) and Leu(309)) that regulate PP2A. In isolated epitrochlearis muscles from rats, GT at 3hPEX with insulin significantly (P less than 0.05) exceeded SED controls. Muscles from 0hPEX vs. 0hSED and 3hPEX vs. 3hSED rats had greater pAS160(Thr642) and pAS160(Ser588). AMPK was the only kinase with greater phosphorylation at 0hPEX vs. 0hSED, and none had greater phosphorylation at 3hPEX vs. 3hSED. Each phosphatase was able to dephosphorylate pAS160(Thr642) and pAS160(Ser588) in cell-free assays. Exercise did not alter posttranslational modifications of PP2A. Our results revealed: 1) pAMPK as a potential trigger for increased pAS160(Thr642) and pAS160(Ser588) at 0hPEX; 2) PP1, PP2A, PP2B, and PP2C were each able to dephosphorylate AS160; and 3) sustained PEX-induced elevations of pAS160(Thr642) and pAS160(Ser588) were attributable to mechanisms other than persistent phosphorylation of known AS160 kinases or altered posttranslational modifications of PP2A. | | 22936728
 |
Hippocampal CA1 transcriptional profile of sleep deprivation: relation to aging and stress.
Porter, NM; Bohannon, JH; Curran-Rauhut, M; Buechel, HM; Dowling, AL; Brewer, LD; Popovic, J; Thibault, V; Kraner, SD; Chen, KC; Blalock, EM
PloS one
7
e40128
2011
Pokaż streszczenie
Many aging changes seem similar to those elicited by sleep-deprivation and psychosocial stress. Further, sleep architecture changes with age suggest an age-related loss of sleep. Here, we hypothesized that sleep deprivation in young subjects would elicit both stress and aging-like transcriptional responses.F344 rats were divided into control and sleep deprivation groups. Body weight, adrenal weight, corticosterone level and hippocampal CA1 transcriptional profiles were measured. A second group of animals was exposed to novel environment stress (NES), and their hippocampal transcriptional profiles measured. A third cohort exposed to control or SD was used to validate transcriptional results with Western blots. Microarray results were statistically contrasted with prior transcriptional studies. Microarray results pointed to sleep pressure signaling and macromolecular synthesis disruptions in the hippocampal CA1 region. Animals exposed to NES recapitulated nearly one third of the SD transcriptional profile. However, the SD-aging relationship was more complex. Compared to aging, SD profiles influenced a significant subset of genes. mRNA associated with neurogenesis and energy pathways showed agreement between aging and SD, while immune, glial, and macromolecular synthesis pathways showed SD profiles that opposed those seen in aging.We conclude that although NES and SD exert similar transcriptional changes, selective presynaptic release machinery and Homer1 expression changes are seen in SD. Among other changes, the marked decrease in Homer1 expression with age may represent an important divergence between young and aged brain response to SD. Based on this, it seems reasonable to conclude that therapeutic strategies designed to promote sleep in young subjects may have off-target effects in the aged. Finally, this work identifies presynaptic vesicular release and intercellular adhesion molecular signatures as novel therapeutic targets to counter effects of SD in young subjects. | | 22792227
 |
Increased expression of serum- and glucocorticoid-regulated kinase-1 in the duodenal mucosa of children with coeliac disease.
Szebeni B, Vannay A, Sziksz E, Prókai A, Cseh A, Veres G, Dezsofi A, Gyorffy H, Szabó IR, Arató A
J Pediatr Gastroenterol Nutr
50
147-53.
2009
Pokaż streszczenie
OBJECTIVES: Enterocyte apoptosis induced by activated intraepithelial lymphocytes is increased in coeliac disease (CD). Serum- and glucocorticoid-regulated kinase-1 (SGK1) is a serine/threonine protein kinase that may inhibit apoptosis and compensate for the excessive death of surface epithelial cells. The significance of SGK1 in CD is elusive so far. The aim of this study was to characterise the expression and localisation of SGK1 in duodenal biopsy samples taken from children with untreated CD, children with treated CD, and controls. | | 19966577
 |
mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1).
Juan M García-Martínez,Dario R Alessi
The Biochemical journal
416
2008
Pokaż streszczenie
SGK1 (serum- and glucocorticoid-induced protein kinase 1) is a member of the AGC (protein kinase A/protein kinase G/protein kinase C) family of protein kinases and is activated by agonists including growth factors. SGK1 regulates diverse effects of extracellular agonists by phosphorylating regulatory proteins that control cellular processes such as ion transport and growth. Like other AGC family kinases, activation of SGK1 is triggered by phosphorylation of a threonine residue within the T-loop of the kinase domain and a serine residue lying within the C-terminal hydrophobic motif (Ser(422) in SGK1). PDK1 (phosphoinositide-dependent kinase 1) phosphorylates the T-loop of SGK1. The identity of the hydrophobic motif kinase is unclear. Recent work has established that mTORC1 [mTOR (mammalian target of rapamycin) complex 1] phosphorylates the hydrophobic motif of S6K (S6 kinase), whereas mTORC2 (mTOR complex 2) phosphorylates the hydrophobic motif of Akt (also known as protein kinase B). In the present study we demonstrate that SGK1 hydrophobic motif phosphorylation and activity is ablated in knockout fibroblasts possessing mTORC1 activity, but lacking the mTORC2 subunits rictor (rapamycin-insensitive companion of mTOR), Sin1 (stress-activated-protein-kinase-interacting protein 1) or mLST8 (mammalian lethal with SEC13 protein 8). Furthermore, phosphorylation of NDRG1 (N-myc downstream regulated gene 1), a physiological substrate of SGK1, was also abolished in rictor-, Sin1- or mLST8-deficient fibroblasts. mTORC2 immunoprecipitated from wild-type, but not from mLST8- or rictor-knockout cells, phosphorylated SGK1 at Ser(422). Consistent with mTORC1 not regulating SGK1, immunoprecipitated mTORC1 failed to phosphorylate SGK1 at Ser(422), under conditions which it phosphorylated the hydrophobic motif of S6K. Moreover, rapamycin treatment of HEK (human embryonic kidney)-293, MCF-7 or HeLa cells suppressed phosphorylation of S6K, without affecting SGK1 phosphorylation or activation. The findings of the present study indicate that mTORC2, but not mTORC1, plays a vital role in controlling the hydrophobic motif phosphorylation and activity of SGK1. Our findings may explain why in previous studies phosphorylation of substrates, such as FOXO (forkhead box O), that could be regulated by SGK, are reduced in mTORC2-deficient cells. The results of the present study indicate that NDRG1 phosphorylation represents an excellent biomarker for mTORC2 activity. | | 18925875
 |
Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor.
Wang, D; Zhang, H; Lang, F; Yun, CC
American journal of physiology. Cell physiology
292
C396-404
2007
Pokaż streszczenie
Glucocorticoids stimulate the intestinal absorption of Na(+) and water partly by regulation of the Na(+)/H(+) exchanger 3 (NHE3). Previous studies have shown both genomic and nongenomic regulation of NHE3 by glucocorticoids. Serum and glucocorticoid-inducible kinase 1 (SGK1) has been shown to be part of this cascade, where phosphorylation of NHE3 by SGK1 initiates the translocation of NHE3 to the cell surface. In the present work, we examined a series of changes in SGK1 and NHE3 induced by glucocorticoids using human colonic Caco-2 and opossum kidney cells. We found that dexamethasone rapidly stimulated SGK1 mRNAs, but a significant change in protein abundance was not detected. Instead, there was an increase in SGK1 kinase activity as early as at 2 h. An increase in NHE3 protein abundance was not detected until 12 h of dexamethasone exposure, although the transport activity was significantly stimulated at 4 h. These data demonstrate that the changes of SGK1 precede those of NHE3. Chronic regulation (24 h) of NHE3 was blocked completely by prevention of protein synthesis with cycloheximide or actinomycin D and by the glucocorticoid receptor blocker RU486. The acute effect of dexamethasone was similarly abrogated by RU486, but was insensitive to cycloheximide and actinomycin D. Similarly, the stimulation of SGK1 activity by dexamethasone was blocked by RU486 but not by actinomycin D. Together, these data show that the acute effect of glucocorticoids on NHE3 is mediated by a glucocorticoid receptor dependent mechanism that activates SGK1 in a nongenomic manner. | Western Blotting | 16971495
 |
SGK protein kinase facilitates the expression of long-term potentiation in hippocampal neurons
Ma, Yun L, et al
Learn Mem, 13:114-8 ()
2005
| | 16585788
 |
Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome.
Nuber, UA; Kriaucionis, S; Roloff, TC; Guy, J; Selfridge, J; Steinhoff, C; Schulz, R; Lipkowitz, B; Ropers, HH; Holmes, MC; Bird, A
Human molecular genetics
14
2247-56
2004
Pokaż streszczenie
Rett syndrome (RTT) is a severe form of mental retardation, which is caused by spontaneous mutations in the X-linked gene MECP2. How the loss of MeCP2 function leads to RTT is currently unknown. Mice lacking the Mecp2 gene initially show normal postnatal development but later acquire neurological phenotypes, including heightened anxiety, that resemble RTT. The MECP2 gene encodes a methyl-CpG-binding protein that can act as a transcriptional repressor. Using cDNA microarrays, we found that Mecp2-null animals differentially express several genes that are induced during the stress response by glucocorticoids. Increased levels of mRNAs for serum glucocorticoid-inducible kinase 1 (Sgk) and FK506-binding protein 51 (Fkbp5) were observed before and after onset of neurological symptoms, but plasma glucocorticoid was not significantly elevated in Mecp2-null mice. MeCP2 is bound to the Fkbp5 and Sgk genes in brain and may function as a modulator of glucocorticoid-inducible gene expression. Given the known deleterious effect of glucocorticoid exposure on brain development, our data raise the possibility that disruption of MeCP2-dependent regulation of stress-responsive genes contributes to the symptoms of RTT. | Western Blotting | 16002417
 |
Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo
Lizcano, J. M., et al
J Biol Chem, 277:27839-49 (2002)
2002
| Immunoblotting (Western) | 12023960
 |
Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase
Kobayashi, T., et al
Biochem J, 344 Pt 1:189-97 (1999)
1998
| Protein Kinase/Phosphatase Assays | 10548550
 |