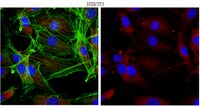
Loss of mTOR signaling affects cone function, cone structure and expression of cone specific proteins without affecting cone survival.
Ma, S; Venkatesh, A; Langellotto, F; Le, YZ; Hall, MN; Rüegg, MA; Punzo, C
Experimental eye research
135
1-13
2015
Pokaż streszczenie
Cones are the primary photoreceptor (PR) cells responsible for vision in humans. They are metabolically highly active requiring phosphoinositide 3-kinase (PI3K) activity for long-term survival. One of the downstream targets of PI3K is the kinase mammalian target of rapamycin (mTOR), which is a key regulator of cell metabolism and growth, integrating nutrient availability and growth factor signals. Both PI3K and mTOR are part of the insulin/mTOR signaling pathway, however if mTOR is required for long-term PR survival remains unknown. This is of particular interest since deregulation of this pathway in diabetes results in reduced PR function before the onset of any clinical signs of diabetic retinopathy. mTOR is found in two distinct complexes (mTORC1 & mTORC2) that are characterized by their unique accessory proteins RAPTOR and RICTOR respectively. mTORC1 regulates mainly cell metabolism in response to nutrient availability and growth factor signals, while mTORC2 regulates pro-survival mechanisms in response to growth factors. Here we analyze the effect on cones of loss of mTORC1, mTORC2 and simultaneous loss of mTORC1 & mTORC2. Interestingly, neither loss of mTORC1 nor mTORC2 affects cone function or survival at one year of age. However, outer and inner segment morphology is affected upon loss of either complex. In contrast, concurrent loss of mTORC1 and mTORC2 leads to a reduction in cone function without affecting cone viability. The data indicates that PI3K mediated pro-survival signals diverge upstream of both mTOR complexes in cones, suggesting that they are independent of mTOR activity. Furthermore, the data may help explain why PR function is reduced in diabetes, which can lead to deregulation of both mTOR complexes simultaneously. Finally, although mTOR is a key regulator of cell metabolism, and PRs are metabolically highly active, the data suggests that the role of mTOR in regulating the metabolic transcriptome in healthy cones is minimal. | | 25887293
 |
Host mTORC1 signaling regulates andes virus replication.
McNulty, S; Flint, M; Nichol, ST; Spiropoulou, CF
Journal of virology
87
912-22
2013
Pokaż streszczenie
Hantavirus pulmonary syndrome (HPS) is a severe respiratory disease characterized by pulmonary edema, with fatality rates of 35 to 45%. Disease occurs following infection with pathogenic New World hantaviruses, such as Andes virus (ANDV), which targets lung microvascular endothelial cells. During replication, the virus scavenges 5'-m(7)G caps from cellular mRNA to ensure efficient translation of viral proteins by the host cell cap-dependent translation machinery. In cells, the mammalian target of rapamycin (mTOR) regulates the activity of host cap-dependent translation by integrating amino acid, energy, and oxygen availability signals. Since there is no approved pharmacological treatment for HPS, we investigated whether inhibitors of the mTOR pathway could reduce hantavirus infection. Here, we demonstrate that treatment with the FDA-approved rapamycin analogue temsirolimus (CCI-779) blocks ANDV protein expression and virion release but not entry into primary human microvascular endothelial cells. This effect was specific to viral proteins, as temsirolimus treatment did not block host protein synthesis. We confirmed that temsirolimus targeted host mTOR complex 1 (mTORC1) and not a viral protein, as knockdown of mTORC1 and mTORC1 activators but not mTOR complex 2 components reduced ANDV replication. Additionally, primary fibroblasts from a patient with tuberous sclerosis exhibited increased mTORC1 activity and increased ANDV protein expression, which were blocked following temsirolimus treatment. Finally, we show that ANDV glycoprotein Gn colocalized with mTOR and lysosomes in infected cells. Together, these data demonstrate that mTORC1 signaling regulates ANDV replication and suggest that the hantavirus Gn protein may modulate mTOR and lysosomal signaling during infection, thus bypassing the cellular regulation of translation. | Western Blotting | 23135723
 |
Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation.
Lu, J; Zavorotinskaya, T; Dai, Y; Niu, XH; Castillo, J; Sim, J; Yu, J; Wang, Y; Langowski, JL; Holash, J; Shannon, K; Garcia, PD
Blood
122
1610-20
2013
Pokaż streszczenie
Multiple myeloma (MM) is the second most common hematologic malignancy. Despite recent treatment advances, it remains incurable. Here, we report that Pim2 kinase expression is highly elevated in MM cells and demonstrate that it is required for MM cell proliferation. Functional interference of Pim2 activity either by short hairpin RNAs or by a potent and selective small-molecule inhibitor leads to significant inhibition of MM cell proliferation. Pim inhibition results in a significant decrease of mammalian target of rapamycin C1 (mTOR-C1) activity, which is critical for cell proliferation. We identify TSC2, a negative regulator of mTOR-C1, as a novel Pim2 substrate and show that Pim2 directly phosphorylates TSC2 on Ser-1798 and relieves the suppression of TSC2 on mTOR-C1. These findings support Pim2 as a promising therapeutic target for MM and define a novel Pim2-TSC2-mTOR-C1 pathway that drives MM proliferation. | Immunoprecipitation | 23818547
 |