Bone morphogenetic protein-2/-4 upregulation promoted by endothelial cells in coculture enhances mouse embryoid body differentiation.
Talavera-Adame, D; Gupta, A; Kurtovic, S; Chaiboonma, KL; Arumugaswami, V; Dafoe, DC
Stem cells and development
22
3252-60
2013
Pokaż streszczenie
Endothelial cells (ECs) provide inductive signals for cell differentiation in vivo. However, it is unknown if these cells promote such differentiation in vitro and the signals involved. We investigated whether ECs are able to enhance the differentiation of the three germ layers and the underlying mechanisms. We established a coculture system of mouse embryoid bodies (EBs) and ECs. Then, we analyzed the expression of markers representative of the three germ layers, such as PDX-1, proinsulin, insulin1 (endoderm), nestin, neurofilament light (ectoderm), CD31, cardiotin, and cardiac troponin I (mesoderm) in EBs cultured alone (controls) or with ECs. A significant increase of these markers was observed in EBs cocultured with ECs compared to controls. The cocultured EBs also exhibited more robust vascular networks similar to those EBs treated with bone morphogenetic protein-2 or -4 (BMP-2 or -4). Therefore, the role of these peptides in the differentiation was investigated. We found a significant upregulation of BMP-2/-4 and BMP receptor 1A in EBs treated with EC conditioned medium (EC-CM) at early or middle stages of EB development. Recombinant human BMP-2 and BMP-4 exerted similar effects than EC-CM in the expression of BMPs or in the upregulation of the three germ layer specific markers. BMP-2/-4 antagonists, such as noggin and chordin-like-1, respectively inhibited the EC-CM inductive effects. These results demonstrate that ECs enhance the differentiation in vitro of cells that derived from the three germ layers and that BMP-2/-4 play a central role in this process. | Immunocytochemistry | 23924071
 |
Plk1 phosphorylation of orc2 and hbo1 contributes to gemcitabine resistance in pancreatic cancer.
Song, B; Liu, XS; Rice, SJ; Kuang, S; Elzey, BD; Konieczny, SF; Ratliff, TL; Hazbun, T; Chiorean, EG; Liu, X
Molecular cancer therapeutics
12
58-68
2013
Pokaż streszczenie
Although gemcitabine is the standard chemotherapeutic drug for treatment of pancreatic cancer, almost all patients eventually develop resistance to this agent. Previous studies identified Polo-like kinase 1 (Plk1) as the mediator of gemcitabine resistance, but the molecular mechanism remains unknown. In this study, we show that Plk1 phosphorylation of Orc2 and Hbo1 mediates the resistance to gemcitabine. We show that the level of Plk1 expression positively correlates with gemcitabine resistance, both in pancreatic cancer cells and xenograft tumors. Overexpression of Plk1 increases gemcitabine resistance, while inhibition of Plk1 sensitizes pancreatic cancer cells to gemcitabine treatment. To validate our findings, we show that inhibition of Plk1 sensitizes tumors to gemcitabine treatment in a mouse xenograft study. Mechanistically, we find that Plk1 phosphorylation of Orc2 maintains DNA replication on gemcitabine treatment. Furthermore, Plk1 phosphorylation of Hbo1 transcriptionally increases cFos expression and consequently elevates its target multidrug resistance 1 (MDR1), which was previously reported to confer chemotherapeutic drug resistance. Knockdown of cFos or MDR1 sensitizes gemcitabine-resistant cells to gemcitabine treatment. Finally, pancreatic cancer cells expressing Plk1-unphosphorylatable mutants of Orc2 or Hbo1 are more sensitive to gemcitabine than cells expressing wild-type Orc2 or Hbo1. In short, our study provides a mechanism for Plk1-mediated gemcitabine resistance, suggesting that Plk1 is a promising target for treatment of gemcitabine-resistant pancreatic cancer. | Western Blotting | 23188630
 |
Endothelial cells in co-culture enhance embryonic stem cell differentiation to pancreatic progenitors and insulin-producing cells through BMP signaling.
Talavera-Adame, D; Wu, G; He, Y; Ng, TT; Gupta, A; Kurtovic, S; Hwang, JY; Farkas, DL; Dafoe, DC
Stem cell reviews
7
532-43
2010
Pokaż streszczenie
Endothelial cells (ECs) represent the major component of the embryonic pancreatic niche and play a key role in the differentiation of insulin-producing β cells in vivo. However, it is unknown if ECs promote such differentiation in vitro. We investigated whether interaction of ECs with mouse embryoid bodies (EBs) in culture promotes differentiation of pancreatic progenitors and insulin-producing cells and the mechanisms involved. We developed a co-culture system of mouse EBs and human microvascular ECs (HMECs). An increase in the expression of the pancreatic markers PDX-1, Ngn3, Nkx6.1, proinsulin, GLUT-2, and Ptf1a was observed at the interface between EBs and ECs (EB-EC). No expression of these markers was found at the periphery of EBs cultured without ECs or those co-cultured with mouse embryonic fibroblasts (MEFs). At EB-EC interface, proinsulin and Nkx6.1 positive cells co-expressed phospho-Smad1/5/8 (pSmad1/5/8). Therefore, EBs were treated with HMEC conditioned media (HMEC-CM) suspecting soluble factors involved in bone morphogenetic protein (BMP) pathway activation. Upregulation of PDX-1, Ngn3, Nkx6.1, insulin-1, insulin-2, amylin, SUR1, GKS, and amylase as well as down-regulation of SST were detected in treated EBs. In addition, higher expression of BMP-2/-4 and their receptor (BMPR1A) were also found in these EBs. Recombinant human BMP-2 (rhBMP-2) mimicked the effects of the HMEC-CM on EBs. Noggin (NOG), a BMP antagonist, partially inhibited these effects. These results indicate that the differentiation of EBs to pancreatic progenitors and insulin-producing cells can be enhanced by ECs in vitro and that BMP pathway activation is central to this process. Pełny tekst artykułu | | 21298405
 |
Poly(ADP-ribose) polymerase-1 (PARP-1) contributes to the barrier function of a vertebrate chromatin insulator.
Aker, M; Bomsztyk, K; Emery, DW
The Journal of biological chemistry
285
37589-97
2009
Pokaż streszczenie
The prototypic chromatin insulator cHS4 has proven effective in reducing silencing chromosomal position effects in a variety of settings. Most of this barrier insulator activity has been mapped to a 250-bp core region, as well as to several proteins that bind this region. However, recent studies from our laboratory demonstrated that an extended 400-bp core region of the cHS4 element is necessary to achieve full barrier insulator activity when used as a single copy in the context of recombinant gammaretroviral and lentiviral vectors. In this study, electrophoretic gel mobility shift assays revealed specific DNA-protein binding activities associated with the distal portion of this extended core region. Affinity purification and tandem mass spectrometry studies led to the identification of one of these proteins as poly(ADP-ribose) polymerase-1 (PARP-1). The identity of this binding activity as PARP-1 was subsequently verified by a variety of biochemical studies in vitro and by chromatin immunoprecipitation studies in vivo. Functional studies with gammaretroviral reporter vectors in cell lines and primary mouse bone marrow progenitor cultures showed that cHS4 barrier activity was abrogated upon mutation of the putative PARP-1-binding site or upon treatment with a PARP inhibitor, respectively. The barrier activity of the cHS4 element was also found to be abrogated in studies using bone marrow from Parp1-null mice. Taken together, this study demonstrates that binding of PARP-1 plays a key functional role in the barrier activity of the extended cHS4 insulator core element. | | 20876582
 |
Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion.
Bauerle KT, Schweppe RE, Haugen BR
Mol Cancer
9
117.
2009
Pokaż streszczenie
BACKGROUND: Nuclear factor-kappaB (NF-kappaB) is constitutively activated in many cancers and plays a key role in promoting cell proliferation, survival, and invasion. Our understanding of NF-kappaB signaling in thyroid cancer, however, is limited. In this study, we have investigated the role of NF-kappaB signaling in thyroid cancer cell proliferation, invasion, and apoptosis using selective genetic inhibition of NF-kappaB in advanced thyroid cancer cell lines. Pełny tekst artykułu | | 20492683
 |
Poly(ADP-ribose) polymerase-1 (PARP-1) is required in murine cell lines for base excision repair of oxidative DNA damage in the absence of DNA polymerase beta.
Le Page, Florence, et al.
J. Biol. Chem., 278: 18471-7 (2003)
2003
Pokaż streszczenie
Oxidative DNA base damage is mainly corrected by the base excision repair (BER) pathway, which can be divided into two subpathways depending on the length of the resynthetized patch, either one nucleotide for short patch BER or several nucleotides for long patch BER. The role of proteins in the course of BER processes has been investigated in vitro using purified enzymes and cell-free extracts. In this study, we have investigated the repair of 8-oxo-7,8-dihydroguanine (8-oxoG) in vivo using wild-type, polymerase beta(-/-) (Polbeta(-/-)), poly(ADP-ribose) polymerase-1(-/-) (PARP-1(-/-)), and Polbeta(-/-)PARP-1(-/-) 3T3 cell lines. We used non replicating plasmids containing a 8-oxoG:C base pair to study the repair of the lesion located in a transcribed sequence (TS) or in a non-transcribed sequence (NTS). The results show that 8-oxoG repair in TS is not significantly impaired in cells deficient in Polbeta or PARP-1 or both. Whereas 8-oxoG repair in NTS is normal in Polbeta-null cells, it is delayed in PARP-1-null cells and greatly impaired in cells deficient in both Polbeta and PARP-1. The removal of 8-oxoG and presumably the cleavage at the resulting apurinic/apyrimidinic site are not affected in the PARP-1(-/-)Polbeta(-/-) cell lines. However, 8-oxoG repair is incomplete, yielding plasmid molecules with a nick at the site of the lesion. Therefore, PARP-1(-/-)Polbeta(-/-) cell lines cannot perform 5'-dRP removal and/or DNA repair synthesis. Furthermore, the poly(ADP-ribosyl)ation activity of PARP-1 is essential for 8-oxoG repair in a Polbeta(-/-) context, because expression of the catalytically inactive PARP-1 (E988K) mutant does not restore 8-oxoG repair, whereas an wild type PARP-1 does. | | 12637553
 |
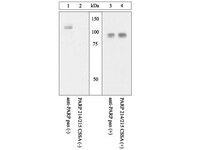
Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update.
Soldani, C and Scovassi, A Ivana
Apoptosis, 7: 321-8 (2002)
2002
Pokaż streszczenie
Poly(ADP-ribosylation) is a post-translational modification of proteins playing a crucial role in many processes, including DNA repair and cell death. The best known poly(ADP-ribosylating) enzyme, PARP-1, is a DNA nick sensor and uses betaNAD(+) to form polymers of ADP-ribose which are further bound to nuclear protein acceptors. To strictly regulate poly(ADP-ribose) turnover, its degradation is assured by the enzyme poly(ADP-ribose) glycohydrolase (PARG). During apoptosis, PARP-1 plays two opposite roles: its stimulation leads to poly(ADP-ribose) synthesis, whereas caspases cause PARP-1 cleavage and inactivation. PARP-1 proteolysis produces an 89 kDa C-terminal fragment, with a reduced catalytic activity, and a 24 kDa N-terminal peptide, which retains the DNA binding domains. The fate and the possible role of these fragments during apoptosis will be discussed. | | 12101391
 |
Model of inhibition of the NPM-ALK kinase activity by herbimycin A.
Turturro, F. et al.
Clin Cancer Res., 8(1):240-5 ()
2002
| | 11801565
 |
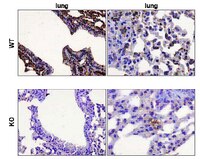
Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice.
Leemans, J.C., et al.
J Immunol., 166(7):4604-11 (2001 )
2001
| | 11254718
 |
Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7.
Germain, M., et al.
J Biol Chem. , 274(40):28379-84 (1999 )
1998
| | 10497198
 |