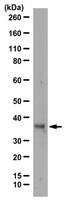
HOXB13 downregulates intracellular zinc and increases NF-κB signaling to promote prostate cancer metastasis.
Kim, YR; Kim, IJ; Kang, TW; Choi, C; Kim, KK; Kim, MS; Nam, KI; Jung, C
Oncogene
33
4558-67
2014
Pokaż streszczenie
Characteristically, prostate cancer (PCa) cells exhibit marked decrease in intracellular zinc; however, the mechanism responsible is not clearly understood. HOXB13 is involved in PCa progression and is overexpressed in castration-resistant PCa. DNA microarray analysis of LNCaP Pca cells showed that ZnT zinc output transporters were strikingly upregulated among androgen-independent HOXB13 target genes. Furthermore, exogenous HOXB13 caused intracellular zinc concentrations to fall in PCa cells, stimulated NF-κB-mediated signaling by reducing inhibitor of NF-κB alpha (IκBα) and enhanced the nuclear translocation of RelA/p65. Human prostate tumors also exhibited strong inverse correlation between the protein expressions of HOXB13 and IκBα. Consequently, HOXB13 stimulated PCa cell invasion, and this was inhibited by the suppression of ZnT4. In addition, studies in a PC3 orthotopic mouse model of PCa metastasis showed that HOXB13 is a strong metastatic stimulator. Taken together, these results show that HOXB13 promotes PCa invasion and metastasis by decreasing intracellular zinc levels, thus stimulating NF-κB signals, and suggest that HOXB13 acts as a modulator of intracellular zinc levels that promotes the malignant characteristics of PCa. | 24096478
 |
HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling.
Kim, YR; Oh, KJ; Park, RY; Xuan, NT; Kang, TW; Kwon, DD; Choi, C; Kim, MS; Nam, KI; Ahn, KY; Jung, C
Molecular cancer
9
124
2009
Pokaż streszczenie
Androgen signaling plays a critical role in the development of prostate cancer and its progression. However, androgen-independent prostate cancer cells emerge after hormone ablation therapy, resulting in significant clinical problems. We have previously demonstrated that the HOXB13 homeodomain protein functions as a prostate cancer cell growth suppressor by inhibiting androgen-mediated signals. However, the role of the HOXB13 in androgen-independent growth of prostate cancer cells remains unexplained.In this report, we first demonstrated that HOXB13 was highly overexpressed in hormone-refractory tumors compared to tumors without prostate-specific antigen after initial treatment. Functionally, in an androgen-free environment minimal induction of HOXB13 in LNCaP prostate cancer cells, to the level of the normal prostate, markedly promoted cell proliferation while suppression inhibited cell proliferation. The HOXB13-mediated cell growth promotion in the absence of androgen, appears to be mainly accomplished through the activation of RB-E2F signaling by inhibiting the expression of the p21waf tumor suppressor. Indeed, forced expression of HOXB13 dramatically decreased expression of p21waf; this inhibition largely affected HOXB13-mediated promotion of E2F signaling.Taken together, the results of this study demonstrated the presence of a novel pathway that helps understand androgen-independent survival of prostate cancer cells. These findings suggest that upregulation of HOXB13 is associated with an additive growth advantage of prostate cancer cells in the absence of or low androgen concentrations, by the regulation of p21-mediated E2F signaling. | 20504375
 |