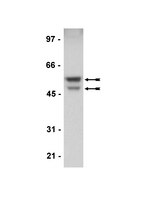
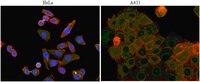
AKT kinase activity is required for lithium to modulate mood-related behaviors in mice.
Pan, JQ; Lewis, MC; Ketterman, JK; Clore, EL; Riley, M; Richards, KR; Berry-Scott, E; Liu, X; Wagner, FF; Holson, EB; Neve, RL; Biechele, TL; Moon, RT; Scolnick, EM; Petryshen, TL; Haggarty, SJ
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology
36
1397-411
2010
Pokaż streszczenie
Bipolar disorder (BP) is a debilitating psychiatric disorder, affecting ∼2% of the worldwide population, for which the etiological basis, pathogenesis, and neurocircuitry remain poorly understood. Individuals with BP suffer from recurrent episodes of mania and depression, which are commonly treated with the mood stabilizer lithium. However, nearly half of BP patients do not respond adequately to lithium therapy and the clinically relevant mechanisms of lithium for mood stabilization remain elusive. Here, we modeled lithium responsiveness using cellular assays of glycogen synthase kinase 3 (GSK-3) signaling and mood-related behavioral assays in inbred strains of mice that differ in their response to lithium. We found that activating AKT through phosphosrylation of a key regulatory site (Thr308) was associated with lithium response-activation of signaling pathways downstream of GSK-3 in cells and attenuation of mood-related behaviors in mice-and this response was attenuated by selective and direct inhibition of AKT kinase activity. Conversely, the expression of constitutively active AKT1 in both the cellular and behavioral assays conferred lithium sensitivity. In contrast, selective and direct GSK-3 inhibition by the ATP-competitive inhibitor CHIR99021 bypassed the requirement for AKT activation and modulated behavior in both lithium-responsive and non-responsive mouse strains. These results distinguish the mechanism of action of lithium from direct GSK-3 inhibition both in vivo and in vitro, and highlight the therapeutic potential for selective GSK-3 inhibitors in BP treatment. | | 21389981
 |
The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth.
Castaño, Zafira, et al.
J. Neurochem., 113: 117-30 (2010)
2009
Pokaż streszczenie
Glycogen synthase kinase-3 (GSK-3) has become an important target for the treatment of mood disorders and neurodegenerative disease. It comprises three enzymes, GSK-3alpha, beta and the neuron-specific isoform, beta2. GSK-3 regulates axon growth by phosphorylating microtubule-associated proteins including Tau. A genetic polymorphism that leads to an increase in the ratio of GSK-3beta1 to GSK-3beta2 interacts with Tau haplotypes to modify disease risk in Parkinson's and Alzheimer's disease. We have examined the roles of each isoform of GSK-3 in neurons. Silencing of GSK-3beta2 inhibited retinoic acid-induced neurite outgrowth in SH-SY5Y neuroblastoma cells and axon growth in rat cortical neurons. Inhibition of neurite outgrowth was prevented by co-expression of GSK-3beta2 but not by co-expression of GSK-3alpha or GSK-3beta1. Ectopic expression GSK-3beta2 enhanced the effects of retinoic acid on neurite length and induced neurite formation in the absence of retinoic acid. GSK-3beta2 phosphorylated Tau at a subset of those sites phosphorylated by GSK-3beta1. In addition, Axin, which regulates responses to Wnt signals, associated more readily with GSK-3beta1 than with GSK-3beta2. Our results suggest that GSK-3 inhibitors that target the Axin-binding site in GSK-3 will preserve the beneficial effects of GSK-3beta2 on axon growth. | | 20067585
 |
Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication.
Chuluunbaatar, U; Roller, R; Feldman, ME; Brown, S; Shokat, KM; Mohr, I
Genes & development
24
2627-39
2009
Pokaż streszczenie
All viruses require cellular ribosomes to translate their mRNAs. Viruses producing methyl-7 (m⁷) GTP-capped mRNAs, like Herpes Simplex Virus-1 (HSV-1), stimulate cap-dependent translation by activating mTORC1 to inhibit the translational repressor 4E-binding protein 1 (4E-BP1). Here, we establish that the HSV-1 kinase Us3 masquerades as Akt to activate mTORC1. Remarkably, Us3 displays no sequence homology with the cellular kinase Akt, yet directly phosphorylates tuberous sclerosis complex 2 (TSC2) on the same sites as Akt. TSC2 depletion rescued Us3-deficient virus replication, establishing that Us3 enhances replication by phosphorylating TSC2 to constitutively activate mTORC1, effectively bypassing S6K-mediated feedback inhibition. Moreover, Us3 stimulated Akt substrate phosphorylation in infected cells, including FOXO1 and GSK3. Thus, HSV-1 encodes an Akt surrogate with overlapping substrate specificity to activate mTORC1, stimulating translation and virus replication. This establishes Us3 as a unique viral kinase with promising drug development potential. | | 21123650
 |
The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth.
Dormoy, V; Danilin, S; Lindner, V; Thomas, L; Rothhut, S; Coquard, C; Helwig, JJ; Jacqmin, D; Lang, H; Massfelder, T
Molecular cancer
8
123
2009
Pokaż streszczenie
Human clear cell renal cell carcinoma (CRCC) remains resistant to therapies. Recent advances in Hypoxia Inducible Factors (HIF) molecular network led to targeted therapies, but unfortunately with only limited clinical significance. Elucidating the molecular processes involved in kidney tumorigenesis and resistance is central to the development of improved therapies, not only for kidney cancer but for many, if not all, cancer types. The oncogenic PI3K/Akt, NF-kB and MAPK pathways are critical for tumorigenesis. The sonic hedgehog (SHH) signaling pathway is crucial to normal development.By quantitative RT-PCR and immunoblot, we report that the SHH signaling pathway is constitutively reactivated in tumors independently of the von Hippel-Lindau (VHL) tumor suppressor gene expression which is inactivated in the majority of CRCC. The inhibition of the SHH signaling pathway by the specific inhibitor cyclopamine abolished CRCC cell growth as assessed by cell counting, BrdU incorporation studies, fluorescence-activated cell sorting and beta-galactosidase staining. Importantly, inhibition of the SHH pathway induced tumor regression in nude mice through inhibition of cell proliferation and neo-vascularization, and induction of apoptosis but not senescence assessed by in vivo studies, immunoblot and immunohistochemistry. Gli1, cyclin D1, Pax2, Lim1, VEGF, and TGF-beta were exclusively expressed in tumors and were shown to be regulated by SHH, as evidenced by immunoblot after SHH inhibition. Using specific inhibitors and immunoblot, the activation of the oncogenic PI3K/Akt, NF-kB and MAPK pathways was decreased by SHH inhibition.These findings support targeting SHH for the treatment of CRCC and pave the way for innovative and additional investigations in a broad range of cancers. | Western Blotting | 20015350
 |
GSK-3 inhibitors: discoveries and developments.
Alonso, M and Martinez, A
Curr. Med. Chem., 11: 755-63 (2004)
2004
Pokaż streszczenie
Glycogen synthase kinase 3 (GSK-3) in the 21(st) century emerged as one of the most attractive therapeutic target for the development of selective inhibitors as new promising drugs for unmet pathologies including Alzheimer's disease, stroke, bipolar disorders, chronic inflammatory processes, cancer and diabetes type II. The full potential of GSK-3 inhibitors is just starting to be realized but the number of candidates in development provided by both academic centres and pharmaceutical companies have increased exponentially in the last two years. This review discloses recent discoveries and developments on peptides and small molecules targeting GSK-3. Focusing attention on this exciting target could thus reap considerable clinical and economic rewards. | | 15032729
 |
GSK-3: tricks of the trade for a multi-tasking kinase.
Doble, Bradley W and Woodgett, James R
J. Cell. Sci., 116: 1175-86 (2003)
2003
Pokaż streszczenie
Glycogen synthase kinase 3 (GSK-3) is a multifunctional serine/threonine kinase found in all eukaryotes. The enzyme is a key regulator of numerous signalling pathways, including cellular responses to Wnt, receptor tyrosine kinases and G-protein-coupled receptors and is involved in a wide range of cellular processes, ranging from glycogen metabolism to cell cycle regulation and proliferation. GSK-3 is unusual in that it is normally active in cells and is primarily regulated through inhibition of its activity. Another peculiarity compared with other protein kinases is its preference for primed substrates, that is, substrates previously phosphorylated by another kinase. Several recent advances have improved our understanding of GSK-3 regulation in multiple pathways. These include the solution of the crystal structure of GSK-3, which has provided insight into GSK-3's penchant for primed substrates and the regulation of GSK-3 by serine phosphorylation, and findings related to the involvement of GSK-3 in the Wnt/beta-catenin and Hedgehog pathways. Finally, since increased GSK-3 activity may be linked to pathology in diseases such as Alzheimer's disease and non-insulin-dependent diabetes mellitus, several new GSK-3 inhibitors, such as the aloisines, the paullones and the maleimides, have been developed. Although they are just starting to be characterized in cell culture experiments, these new inhibitors hold promise as therapeutic agents. | | 12615961
 |
Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes.
Jope, Richard S
Trends Pharmacol. Sci., 24: 441-3 (2003)
2003
Pokaż streszczenie
The intrigue of lithium, the simplest drug in the modern pharmacopoeia, extends from its complex actions in cells to its therapeutic effects as a mood stabilizer. New surprises from studies of glycogen synthase kinase 3 (GSK-3) show that lithium reduces GSK-3 activity in two ways, both directly and by increasing the inhibitory phosphorylation of GSK-3. These dual effects can act in concert to magnify the influence of lithium on crucial GSK-3-regulated functions (gene expression, cell structure and survival). | | 12967765
 |