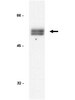
FOXO3A directs a protective autophagy program in haematopoietic stem cells.
Warr, MR; Binnewies, M; Flach, J; Reynaud, D; Garg, T; Malhotra, R; Debnath, J; Passegué, E
Nature
494
323-7
2013
Pokaż streszczenie
Blood production is ensured by rare, self-renewing haematopoietic stem cells (HSCs). How HSCs accommodate the diverse cellular stresses associated with their life-long activity remains elusive. Here we identify autophagy as an essential mechanism protecting HSCs from metabolic stress. We show that mouse HSCs, in contrast to their short-lived myeloid progeny, robustly induce autophagy after ex vivo cytokine withdrawal and in vivo calorie restriction. We demonstrate that FOXO3A is critical to maintain a gene expression program that poises HSCs for rapid induction of autophagy upon starvation. Notably, we find that old HSCs retain an intact FOXO3A-driven pro-autophagy gene program, and that ongoing autophagy is needed to mitigate an energy crisis and allow their survival. Our results demonstrate that autophagy is essential for the life-long maintenance of the HSC compartment and for supporting an old, failing blood system. | 23389440
 |
Platelet-derived growth factor-A regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a.
McGowan, SE; McCoy, DM
Respiratory research
14
68
2013
Pokaż streszczenie
Secondary pulmonary alveolar septal formation requires platelet derived growth factor (PDGF-A) and platelet derived growth factor receptor-alpha (PDGFRα), and their regulation influences alveolar septal areal density and thickness. Insufficient PDGFRα expression in lung fibroblasts (LF) results in failed septation.Mice in which the endogenous PDGFRα-gene regulates expression of the green fluorescent protein were used to temporally and spatially track PDGFRα-signaling. Transition from the G₁/G₀ to the S-phase of the cell cycle was compared in PDGFRα-expressing and non-expressing LF using flow cytometry. Laser scanning confocal microscopy was used to quantify p27(kip1) and forkhead box "other" 3a (FoxO3a) in the nuclei of alveolar cells from mice bearing the PDGFRα-GFP knock-in, and p27(kip1) in mice with a conditional deletion of PDGFRα-gene function. The effects of PDGF-A on the phosphorylation and the intracellular location of FoxO3a were examined using Western immuoblotting and immunocytochemistry.In neonatal mouse lungs, entry of the PDGFRα-expressing LF subpopulation into the S-phase of the cell cycle diminished sooner than in their non-expressing LF counterparts. This preferential diminution was influenced by PDGFRα-mediated signaling, which phosphorylates and promotes cytoplasmic localization of FoxO3a. Comparative observations of LF at different ages during secondary septation and in mice that lack PDGFRα in alveolar LF demonstrated that nuclear localization of the G₁ cyclin-dependent kinase inhibitor p27(kip1) correlated with reduced LF entry into S-phase.Nuclear localization of FoxO3a, an important regulator of p27(kip1) gene-expression, correlates with diminished proliferation of the PDGFRα-expressing LF subpopulation. These mechanisms for diminishing the effects of PDGFRα-mediated signaling likely regulate secondary septal formation and their derangement may contribute to imbalanced fibroblast cell kinetics in parenchymal lung diseases. | 23819440
 |
The E-box binding factors Max/Mnt, MITF, and USF1 act coordinately with FoxO to regulate expression of proapoptotic and cell cycle control genes by phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3 signaling.
Terragni, J; Nayak, G; Banerjee, S; Medrano, JL; Graham, JR; Brennan, JF; Sepulveda, S; Cooper, GM
The Journal of biological chemistry
286
36215-27
2010
Pokaż streszczenie
Phosphatidylinositol (PI) 3-kinase/Akt signaling plays a critical role in cell proliferation and survival, partly by regulation of FoxO transcription factors. Previous work using global expression profiling indicated that inhibition of PI 3-kinase in proliferating cells led to induction of genes that promote cell cycle arrest and apoptosis. The upstream regulatory regions of these genes had binding sites not only for FoxO, but also for Myc/Max transcription factors. In the present study, we have addressed the role of Myc family members and related E-box-binding proteins in the regulation of these genes. Chromatin immunoprecipitations and RNA interference indicated that transcription was repressed by Max-Mnt-Sin3a-histone deacetylase complexes in proliferating cells. Inhibition of PI 3-kinase led to a loss of Max/Mnt binding and transcriptional induction by MITF and USF1, as well as FoxO. Both MITF and USF1 were activated by glycogen synthase kinase (GSK) 3, with GSK3 phosphorylation sites on USF1 identified as the previously described activating site threonine 153 as well as serine 186. siRNA against MITF as well as against FoxO3a protected cells from apoptosis following PI 3-kinase inhibition. These results define a novel E-box-regulated network that functions coordinately with FoxO to regulate transcription of apoptotic and cell cycle regulatory genes downstream of PI 3-kinase/Akt/GSK3 signaling. | 21873430
 |















-(+)-Glutamine[816016_(S)-(+)-Glutamine-ALL].jpg)