Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors.
Husak, Z; Printz, D; Schumich, A; Pötschger, U; Dworzak, MN
Journal of leukocyte biology
88
405-12
2009
Pokaż streszczenie
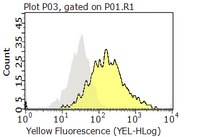
Our study was performed to examine the role of CD99 in normal and leukemia BCPs. CD99 is strongly expressed by certain pediatric cancers including BCP-ALL. Modulation of the antigen in ETs and T cells induces apoptosis, hence implicating CD99 as a potential target for anti-cancer therapy. However, nothing is known about these aspects in BCPs. We investigated BCP-ALL cases and normal BCP cells from pediatric BM for CD99 protein and RNA expression as well as for effects of CD99 modulation by mAb. Immunophenotypes, recovery, apoptosis, and aggregation were assessed. Flow cytometry, light microscopy, and qRT-PCR were used in our experiments. An association of CD99 expression levels with the cytogenetic background of pediatric BCP-ALLs was found. Highest CD99 levels were observed in hyperdiploid, followed by TEL/AML1 and random karyotype leukemias. CD99 ligation moderately induced cell death only in TEL/AML1 cases. Stroma cell contact mitigated this effect. Very immature normal BCPs were the most sensitive to CD99-mediated death induction. Type I CD99 mRNA was the main isoform in ALLs and was expressed differentially during BCP maturation. Our data suggest that clinical targeting of CD99 may be effective in BCP-ALL-bearing TEL/AML1 but also may elicit negative effects on normal B-lymphopoiesis. We consider our results as an indication that CD99 may play a physiologic role in the clonal deletion processes necessary for B-lymphoid selection. | 20453109
 |
CD99 expressed on human mobilized peripheral blood CD34+ cells is involved in transendothelial migration.
Imbert, AM; Belaaloui, G; Bardin, F; Tonnelle, C; Lopez, M; Chabannon, C
Blood
108
2578-86
2005
Pokaż streszczenie
Hematopoietic progenitor cell trafficking is an important phenomenon throughout life. It is thought to occur in sequential steps, similar to what has been described for mature leukocytes. Molecular actors have been identified for each step of leukocyte migration; recently, CD99 was shown to play a part during transendothelial migration. We explored the expression and role of CD99 on human hematopoietic progenitors. We demonstrate that (1) CD34+ cells express CD99, albeit with various intensities; (2) subsets of CD34+ cells with high or low levels of CD99 expression produce different numbers of erythroid, natural killer (NK), or dendritic cells in the in vitro differentiation assays; (3) the level of CD99 expression is related to the ability to differentiate toward B cells; (4) CD34+ cells that migrate through an endothelial monolayer in response to SDF-1alpha and SCF display the highest level of CD99 expression; (5) binding of a neutralizing antibody to CD99 partially inhibits transendothelial migration of CD34+ progenitors in an in vitro assay; and (6) binding of a neutralizing antibody to CD99 reduces homing of CD34+ progenitors xenotransplanted in NOD-SCID mice. We conclude that expression of CD99 on human CD34+ progenitors has functional significance and that CD99 may be involved in transendothelial migration of progenitors. | 16825498
 |
Recognition of Mycoplasma hyorhinis by CD99-Fc molecule.
Gazit, R; Rechnitzer, H; Achdout, H; Katzenell, A; Katz, G; Markel, G; Arnon, TI; Gonen-Gross, T; Mizrahi, S; Gruda, R; Rottem, S; Mandelboim, O
European journal of immunology
34
2032-40
2004
Pokaż streszczenie
The human CD99 protein is expressed on many cell types and is mostly abundant on lymphocytes and on several tumors. Different functions were attributed to the CD99 receptor, including adhesion, apoptosis and activation. However, until now the only ligand suggested to be recognized by CD99 was CD99 itself. In order to identify possible new CD99 ligands we constructed a CD99 protein fused to human IgG1. Surprisingly, a pronounced specific staining of melanoma cell lines that were infected with mycoplasmas was observed whereas clean cells were not recognized. Staining was specific, as other fusion proteins did not recognize the mycoplasma-infected cells. Sequencing of the 23s-16s region revealed that the contaminating agent is Mycoplasma hyorhinis. The CD99 interaction with M. hyorhinis was direct since it was blocked by anti-CD99 monoclonal antibody and by M. hyorhinis. It was also strain-specific as other mycoplasmas were not recognized. Our results show that CD99 interacts with a novel ligand of M. hyorhinis. | 15214051
 |
CD99 plays a major role in the migration of monocytes through endothelial junctions.
Schenkel, AR; Mamdouh, Z; Chen, X; Liebman, RM; Muller, WA
Nature immunology
3
143-50
2002
Pokaż streszczenie
CD99 is a heavily O-glycosylated 32-kD type I transmembrane protein that is expressed on most hematopoietic cells. We show here that CD99 is expressed on endothelial cells and is concentrated at the borders between confluent cells. We found that a monoclonal antibody to CD99, hec2, selectively inhibited diapedesis of monocytes across endothelial cells by >90%. Diapedesis involved the homophilic interaction of CD99 on monocytes with CD99 on endothelial junctions. CD99 functioned distally to the point at which platelet-endothelial cell adhesion molecule 1 (PECAM-1, also known as CD31), another adhesion molecule involved in transmigration, played its critical role. Confocal microscopy showed that anti-PECAM-1 arrested leukocytes on the apical surface of endothelium, whereas blocking CD99 arrested monocytes at a point where they were partially through the junction. Therefore, diapedesis, the forward migration of leukocytes through endothelial junctions, is regulated sequentially by two distinct molecules, PECAM-1 and CD99. | 11812991
 |
The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes.
Bernard, G; Zoccola, D; Deckert, M; Breittmayer, JP; Aussel, C; Bernard, A
Journal of immunology (Baltimore, Md. : 1950)
154
26-32
1994
Pokaż streszczenie
We have previously described E2 as a 32-kDa transmembrane glycoprotein displaying an isomorphism, as two epitopes (defined by mAbs O662 and L129) are widely distributed on T cells whereas two epitopes are restricted to T cell subsets (defined by mAbs D44 and 12E7). E2, the MIC-2 gene product, is involved in T cell adhesion because anti-E2 mAbs against pan T epitopes block spontaneous T cell rosettes. Pan T E2 mAbs are also able to induce exposure of the phosphatidylserine at the thymocyte surface but not at the surface of mature T lymphocytes, an event most likely linked to adhesion phenomena. We now show here that the anti-E2 mAbs (0662 and L129) that block rosettes and induce phosphatidylserine exposure at the thymocyte surface, and not those reacting with epitopes not involved in adhesion, also trigger aggregation of certain immature T cell lines and no other cell lines tested. Among the normal cells tested, anti-E2 mAbs exclusively induce homotypic aggregation of CD4+ CD8+ human thymocytes. This phenomenon is temperature, energy, and Mg++ dependent, and requires an intact cytoskeleton. These adhesion properties are rather characteristic of integrins. Nevertheless, mAb against beta 1, beta 2, and beta 3 integrin chains, as well as those against alpha-chains known to be present on thymocytes, are unable to block corticothymocyte aggregation. We conclude that E2 triggers on corticothymocytes and no other T cells a homotypic adhesion pathway most likely mediated by an uncharacterized integrin. | 7527813
 |
The E2 antigen, a 32 kd glycoprotein involved in T-cell adhesion processes, is the MIC2 gene product.
Gelin, C; Aubrit, F; Phalipon, A; Raynal, B; Cole, S; Kaczorek, M; Bernard, A
The EMBO journal
8
3253-9
1988
Pokaż streszczenie
E2 is a 32 kd human T-cell surface glycoprotein involved in spontaneous rosette formation with erythrocytes. A 1.11 kb cDNA was isolated from a lambda gt11 expression library by screening with monoclonal antibodies directed against E2. The primary structure of E2, deduced from the nucleotide sequence of its gene, comprises 185 amino acids and is devoid of N-linked glycosylation sites. The E2 protein is rich in proline residues and displays an organization typical of an integral membrane protein. Northern blotting showed a good correlation between mRNA abundance, E2 surface density and the level of T cell differentiation. In fact, nucleotide sequencing revealed that E2 is the MIC2 gene product, previously identified with the 12E7 Mab. Xg(a-) female individuals have no E2 molecule on the surface of their red cells, in contrast with Xg(a+) individuals, but have the molecule in their cytoplasm, in the form of the 28 kd precursor. These findings show that the E2 antigen, a cell surface molecule involved in T cell adhesion processes, is the product of the MIC2 gene, the only pseudoautosomal gene to be described in man. | 2479542
 |