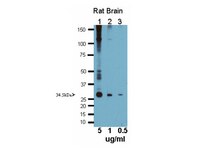
Evidence of a rudimentary colon in the elasmobranch, Leucoraja erinacea.
Nicole Alexandra Theodosiou,Alyssa Simeone
Development genes and evolution
222
2011
Pokaż streszczenie
The transition from aquatic to terrestrial life presented tetrapodamorphs with the challenge of maintaining water homeostasis and preventing desiccation on land. The colon evolved in terrestrial vertebrates to help maintain fluid balance. Although marine elasmobranchs lack a colon, their spiral intestine contains a subregion that histologically appears to be colon-like, possibly representing an evolutionary precursor to terrestrial digestive tracts. The distal-most region of the spiral intestine of elasmobranchs has no villi and a large number of acid mucins: hallmarks of water absorption in the colons of terrestrial animals. To determine if histologically distinct regions of the elasmobranch digestive tract correspond to functional differences, we compared water absorption in different subregions of the skate, Leucoraja erinacea digestive tract. Water absorption in stomach and spiral intestinal sacs was linear with time and not hydrostatic pressure-dependent. The histologically distinct distal portion of the spiral intestine had a threefold higher rate of water absorption than the proximal portion of the spiral intestine. In addition, the water-selective, colon-specific aquaporin 4 is expressed strongly in the distal spiral intestine epithelia, correlating with the region of the spiral intestine exhibiting the greatest rate of water absorption. We demonstrate that the distal spiral intestine is histologically and functionally distinct from the rest of the spiral intestine and represents a rudimentary colon within the vertebrate lineage. | 22610344
 |
Absence of glial α-dystrobrevin causes abnormalities of the blood-brain barrier and progressive brain edema.
Lien, CF; Mohanta, SK; Frontczak-Baniewicz, M; Swinny, JD; Zablocka, B; Górecki, DC
The Journal of biological chemistry
287
41374-85
2011
Pokaż streszczenie
The blood-brain barrier (BBB) plays a key role in maintaining brain functionality. Although mammalian BBB is formed by endothelial cells, its function requires interactions between endotheliocytes and glia. To understand the molecular mechanisms involved in these interactions is currently a major challenge. We show here that α-dystrobrevin (α-DB), a protein contributing to dystrophin-associated protein scaffolds in astrocytic endfeet, is essential for the formation and functioning of BBB. The absence of α-DB in null brains resulted in abnormal brain capillary permeability, progressively escalating brain edema, and damage of the neurovascular unit. Analyses in situ and in two-dimensional and three-dimensional in vitro models of BBB containing α-DB-null astrocytes demonstrated these abnormalities to be associated with loss of aquaporin-4 water and Kir4.1 potassium channels from glial endfeet, formation of intracellular vacuoles in α-DB-null astrocytes, and defects of the astrocyte-endothelial interactions. These caused deregulation of tight junction proteins in the endothelia. Importantly, α-DB but not dystrophins showed continuous expression throughout development in BBB models. Thus, α-DB emerges as a central organizer of dystrophin-associated protein in glial endfeet and a rare example of a glial protein with a role in maintaining BBB function. Its abnormalities might therefore lead to BBB dysfunction. | 23043099
 |
Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation.
Esther Steiner,Gaby U Enzmann,Shuo Lin,Sharang Ghavampour,Melanie-Jane Hannocks,Benoît Zuber,Markus A Rüegg,Lydia Sorokin,Britta Engelhardt
Glia
60
2011
Pokaż streszczenie
Brain edema is the main cause of death from brain infarction. The polarized expression of the water channel protein aquaporin-4 (AQP4) on astroglial endfeet surrounding brain microvessels suggests a role in brain water balance. Loss of astrocyte foot process anchoring to the basement membrane (BM) accompanied by the loss of polarized localization of AQP4 to astrocytic endfeet has been shown to be associated with vasogenic/extracellular edema in neuroinflammation. Here, we asked if loss of astrocyte polarity is also observed in cytotoxic/intracellular edema following focal brain ischemia after transient middle cerebral artery occlusion (tMCAO). Upon mild focal brain ischemia, we observed diminished immunostaining for the BM components laminin α4, laminin α2, and the proteoglycan agrin, in the core of the lesion, but not in BMs in the surrounding penumbra. Staining for the astrocyte endfoot anchorage protein β-dystroglycan (DG) was dramatically reduced in both the lesion core and the penumbra, and AQP4 and Kir4.1 showed a loss of polarized localization to astrocytic endfeet. Interestingly, we observed that mice deficient for agrin expression in the brain lack polarized localization of β-DG and AQP4 at astrocytic endfeet and do not develop early cytotoxic/intracellular edema following tMCAO. Taken together, these data indicate that the binding of DG to agrin embedded in the subjacent BM promotes polarized localization of AQP4 to astrocyte endfeet. Reduced DG protein levels and redistribution of AQP4 as observed upon tMCAO might therefore counteract early edema formation and reflect a beneficial mechanism operating in the brain to minimize damage upon ischemia. © 2012 Wiley Periodicals, Inc. | 22782669
 |
Fluid-percussion brain injury induces changes in aquaporin channel expression.
Oliva AA Jr, Kang Y, Truettner JS, Sanchez-Molano J, Furones C, Yool AJ, Atkins CM
Neuroscience
2010
Pokaż streszczenie
Edema, the accumulation of excess fluid, is a major pathological change in the brain that contributes significantly to pathology and mortality after moderate to severe brain injury. Edema is regulated by aquaporin (AQP) channels which transport water across cellular membranes. Six AQPs are found in the brain (1, 3, 4, 5, 8, and 9), and previous studies have found that AQP4 is regulated after traumatic brain injury (TBI). To further understand how AQPs contribute to brain edema, we investigated whether expression of AQP1, 3, and 9 are also regulated after TBI. Adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury (FPI) or sham surgery. After induction of FPI, the injured, ipsilateral parietal cortex and hippocampus were dissected and analyzed by Western blotting. We observed a small decrease in AQP3 and 4 levels at 7 days after FPI in the ipsilateral, parietal cortex. Both AQP1 and 9 significantly increased within 30 min post-injury and remained elevated for up to 6 h in the ipsilateral, parietal cortex. Aqp1 and 9 mRNA levels were also significantly increased at 30 min post-FPI. Administration of an AQP1 and 4 antagonist, AqB013, non-significantly increased brain water content in sham, non-injured animals, and did not prevent edema formation 24 h after trauma in either the parietal cortex or hippocampus. These results indicate that Aqp1 and 9 mRNA and protein levels increase after moderate parasagittal FPI and that an inhibitor of AQP1 and 4 does not decrease edema after moderate parasagittal FPI.Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved. | 21329742
 |
Extracellular DNA traps promote thrombosis.
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD
Proc Natl Acad Sci U S A
107
15880-5. Epub 2010 Aug 23.
2009
Pokaż streszczenie
Neutrophil extracellular traps (NETs) are part of the innate immune response to infections. NETs are a meshwork of DNA fibers comprising histones and antimicrobial proteins. Microbes are immobilized in NETs and encounter a locally high and lethal concentration of effector proteins. Recent studies show that NETs are formed inside the vasculature in infections and noninfectious diseases. Here we report that NETs provide a heretofore unrecognized scaffold and stimulus for thrombus formation. NETs perfused with blood caused platelet adhesion, activation, and aggregation. DNase or the anticoagulant heparin dismantled the NET scaffold and prevented thrombus formation. Stimulation of platelets with purified histones was sufficient for aggregation. NETs recruited red blood cells, promoted fibrin deposition, and induced a red thrombus, such as that found in veins. Markers of extracellular DNA traps were detected in a thrombus and plasma of baboons subjected to deep vein thrombosis, an example of inflammation-enhanced thrombosis. Our observations indicate that NETs are a previously unrecognized link between inflammation and thrombosis and may further explain the epidemiological association of infection with thrombosis. Pełny tekst artykułu | 20798043
 |
Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury.
Marignier, R; Nicolle, A; Watrin, C; Touret, M; Cavagna, S; Varrin-Doyer, M; Cavillon, G; Rogemond, V; Confavreux, C; Honnorat, J; Giraudon, P
Brain : a journal of neurology
133
2578-91
2009
Pokaż streszczenie
Devic's neuromyelitis optica is an inflammatory demyelinating disorder normally restricted to the optic nerves and spinal cord. Since the identification of a specific autoantibody directed against aquaporin 4, neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody, neuromyelitis optica has been considered an entity distinct from multiple sclerosis. Recent findings indicate that the neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody has a pathogenic role through complement-dependent astrocyte toxicity. However, the link with demyelination remains elusive. Autoantibodies can act as receptor agonists/antagonists or alter antigen density in their target cells. We hypothesized that the neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody impairs astrocytic function and secondarily leads to demyelination. Rat astrocytes and oligodendrocytes from primary cultures and rat optic nerves were exposed long-term (24 h) to immunoglobulin G in the absence of complement. Immunoglobulin G was purified from the serum of patients with neuromyelitis optica who were either neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody positive or negative, as well as from healthy controls. Flow cytometry analysis showed a reduction of membrane aquaporin 4 and glutamate transporter type 1 on astrocytes following contact with immunoglobulin G purified from neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody positive serum only. The activity of glutamine synthetase, an astrocyte enzyme converting glutamate into glutamine, decreased in parallel, indicating astrocyte dysfunction. Treatment also reduced oligodendrocytic cell processes and approximately 30% oligodendrocytes died. This deleterious effect was confirmed ex vivo; exposed optic nerves showed reduction of myelin basic protein. Immunoglobulin G from neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody seronegative patients and from healthy controls had no similar effect. Neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody did not directly injure oligodendrocytes cultured without astrocytes. A toxic bystander effect of astrocytes damaged by neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody on oligodendrocytes was identified. Progressive accumulation of glutamate in the culture medium of neuromyelitis optica-immunoglobulin G/aquaporin 4-antibody-treated glial cells supported the hypothesis of a glutamate-mediated excitotoxic death of oligodendrocytes in our models. Moreover, co-treatment of glial cultures with neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody and d+2-amino-5-phosphonopentanoic acid, a competitive antagonist at the N-methyl-d-aspartate/glutamate receptor, partially protected oligodendrocytes. Co-immunolabelling of oligodendrocyte markers and neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody showed that astrocytic positive processes were in close contact with oligodendrocytes and myelin in rat optic nerves and spinal cord, but far less so in other parts of the central nervous system. This suggests a bystander effect of neuromyelitis optica-immunoglobulin G-damaged astrocytes on oligodendrocytes in the nervous tissues affected by neuromyelitis optica. In conclusion, in these cell culture models we found a direct, complement-independent effect of neuromyelitis optica-immunoglobulin G/aquaporin 4 antibody on astrocytes, with secondary damage to oligodendrocytes possibly resulting from glutamate-mediated excitotoxicity. These mechanisms could add to the complement-induced damage, particularly the demyelination, seen in vivo. | 20688809
 |
Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK.
Murase T, Misawa K, Haramizu S, Minegishi Y, Hase T
Am J Physiol Endocrinol Metab
299
E266-75. Epub 2010 May 25.
2009
Pokaż streszczenie
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is implicated in the control of energy metabolism and is considered to be a molecular target for the suppression of obesity and the treatment of metabolic syndrome. Here, we identified and characterized nootkatone, a constituent of grapefruit, as a naturally occurring AMPK activator. Nootkatone induced an increase in AMPKalpha1 and -alpha2 activity along with an increase in the AMP/ATP ratio and an increase the phosphorylation of AMPKalpha and the downstream target acetyl-CoA carboxylase (ACC), in C(2)C(12) cells. Nootkatone-induced activation of AMPK was possibly mediated both by LKB1 and Ca(2+)/calmodulin-dependent protein kinase kinase. Nootkatone also upregulated PPARgamma coactivator-1alpha in C(2)C(12) cells and C57BL/6J mouse muscle. In addition, administration of nootkatone (200 mg/kg body wt) significantly enhanced AMPK activity, accompanied by LKB1, AMPK, and ACC phosphorylation in the liver and muscle of mice. Whole body energy expenditure evaluated by indirect calorimetry was also increased by nootkatone administration. Long-term intake of diets containing 0.1% to 0.3% (wt/wt) nootkatone significantly reduced high-fat and high-sucrose diet-induced body weight gain, abdominal fat accumulation, and the development of hyperglycemia, hyperinsulinemia, and hyperleptinemia in C57BL/6J mice. Furthermore, endurance capacity, evaluated as swimming time to exhaustion in BALB/c mice, was 21% longer in mice fed 0.2% nootkatone than in control mice. These findings indicate that long-term intake of nootkatone is beneficial toward preventing obesity and improving physical performance and that these effects are due, at least in part, to enhanced energy metabolism through AMPK activation in skeletal muscle and liver. | 20501876
 |
Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis.
Karen Wolburg-Buchholz,Andreas F Mack,Esther Steiner,Friederike Pfeiffer,Britta Engelhardt,Hartwig Wolburg
Acta neuropathologica
118
2009
Pokaż streszczenie
In multiple sclerosis (MS), and its animal model experimental autoimmune encephalomyelitis (EAE), dysfunction of the blood-brain barrier (BBB) leads to edema formation within the central nervous system. The molecular mechanisms of edema formation in EAE/MS are poorly understood. We hypothesized that edema formation is due to imbalanced water transport across the BBB caused by a disturbed crosstalk between BBB endothelium and astrocytes. Here, we demonstrate at the light microscopic and ultrastructural level, the loss of polarized localization of the water channel protein aquaporin-4 (AQP4) in astrocytic endfeet surrounding microvessels during EAE. AQP4 was found to be redistributed over the entire astrocytic cell surface and lost its arrangement in orthogonal arrays of intramembranous particles as seen in the freeze-fracture replica. In addition, immunostaining for the astrocytic extracellular matrix receptor beta-dystroglycan disappeared from astroglial membranes in the vicinity of inflammatory cuffs, whereas immunostaining for the dystroglycan ligands agrin and laminin in the perivascular basement membrane remained unchanged. Our data suggest that during EAE, loss of beta-dystroglycan-mediated astrocyte foot process anchoring to the basement membrane leads to loss of polarized AQP4 localization in astrocytic endfeet, and thus to edema formation in EAE. | 19533155
 |