505763 Sigma-AldrichProRS Inhibitor, Halofuginone - CAS 64924-67-0 - Calbiochem
A cell-permeable racemic mixture of Halofuginone that inhibits of prolyl-tRNA synthetase.
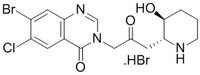
More>> A cell-permeable racemic mixture of Halofuginone that inhibits of prolyl-tRNA synthetase. Less<<Synonyms: STENOROL, HF, Hydrobromide, prolyl-tRNA synthetase Inhibitor, trans-(±)-7-Bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone hydrobromide
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 64924-67-0 | C₁₆H₁₇BrClN₃O₃•HBr |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.05763.0001 | Glass bottle | 10 mg |
| Product Information | |
|---|---|
| CAS number | 64924-67-0 |
| Form | White powder |
| Hill Formula | C₁₆H₁₇BrClN₃O₃•HBr |
| Chemical formula | C₁₆H₁₇BrClN₃O₃•HBr |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | prolyl-tRNA synthetase |
| Primary Target IC<sub>50</sub> | 18 nM |
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.05763.0001 | 04055977243536 |
Documentation
ProRS Inhibitor, Halofuginone - CAS 64924-67-0 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Zhou, H., et al. 2013, Nature 494, 121. Keller, T.L., et al. 2012, Nat. Chem. Biol. 12, 311. Sundrud, M.S., et al. 2009, Science 324, 1334. Elkin, M., et al. 2000, FASEB J. 14, 2477. Elkin, M., et al. 1999, Clin. Cancer Res. 5, 1982. |