513028 Sigma-AldrichPD 166285 - CAS 212391-63-4 - Calbiochem
A cell-permeable, orally bioavailable, ATP-competitive, broad-spectrum tyrosine kinase inhibitor that suppresses angiogenesis both in vitro and in vivo.
More>> A cell-permeable, orally bioavailable, ATP-competitive, broad-spectrum tyrosine kinase inhibitor that suppresses angiogenesis both in vitro and in vivo. Less<<Synonyms: PD166285, PD0166285, 6-(2,6-Dichlorophenyl)-2-(4-(2-(diethylaminoethoxy)-phenylamino)-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one, 2HCl, Wee1 Inhibitor IV, PDGFR Tyrosine Kinase Inhibitor XIX
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
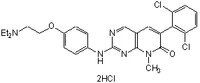
| 212391-63-4 | C₂₆H₂₇Cl₂N₅O₂ • 2HCl |
| Description | |
|---|---|
| Overview | A cell-permeable, orally bioavailable, ATP-competitive, broad-spectrum tyrosine kinase inhibitor (IC50 against against c-Src, Wee1, FGFR-1, Myt1, EGFR, and PDGFRβ = 8.4, 24, 39.3, 72, 87.5 and 98.3 nM, respectively) that suppresses angiogenesis both in vitro (max inhibition dose at 100 nM in HUVEC microcapillary formation assays) and in vivo (max inhibition achieved via 5 mg/kg p.o. in murine Matrigel plug angiogenesis assays), while exhibiting much reduced potency against Chk1, MAPK, and PKC (IC50 = 3.4, 5, and 22.7 µM, respectively) and little activity toward IRTK and Cdk4/D1 even at concentrations as high as 50 µM. Shown to effectively block PDGF-, EGF-, and bFGF-stimulated receptor phosphorylations (IC50 = 6.5, 1600, and 97.3 nM, respectively) and other cellular responses in rat aortic smooth muscle cells. Inhibition of cellular Wee1 activity by 500 nM PD 166285 in combination with 50 ng/ml nocodazole (Cat. No. 487928) treatment is also reported to result in a blockage of radiation-induced Cdc2 phosphorylation on Tyr15 and Thr14 in 7 human cancer cells and specifically demonstrated to sensatize PA-1 cultures to radiation-induced cell death in a p53-dependent manner. |
| Catalogue Number | 513028 |
| Brand Family | Calbiochem® |
| Synonyms | PD166285, PD0166285, 6-(2,6-Dichlorophenyl)-2-(4-(2-(diethylaminoethoxy)-phenylamino)-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one, 2HCl, Wee1 Inhibitor IV, PDGFR Tyrosine Kinase Inhibitor XIX |
| Product Information | |
|---|---|
| CAS number | 212391-63-4 |
| Form | Pale yellow solid |
| Hill Formula | C₂₆H₂₇Cl₂N₅O₂ • 2HCl |
| Chemical formula | C₂₆H₂₇Cl₂N₅O₂ • 2HCl |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 513028 | 0 |
Documentation
PD 166285 - CAS 212391-63-4 - Calbiochem SDS
| Title |
|---|
PD 166285 - CAS 212391-63-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 513028 |
References
| Reference overview |
|---|
| Wang, Y., et al. 2001. Cancer Res. 61, 8211. Dimitroff, C.J., et al. 1999. Invest. New Drugs 17, 121. Roginskaya, V., et al. 1999. Leukemia 13, 855. Panek, R.L., et al. 1997. J. Pharmacol. Exp. Ther. 283, 1433. |