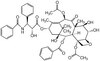
110110 N-Acetyl-S-farnesyl-L-cysteine
Recommended Products
Overview
| Replacement Information |
|---|
| Product Information | |
|---|---|
| CAS number | 135304-07-3 |
| Form | Clear oil |
| Hill Formula | C₂₀H₃₃NO₃S |
| Chemical formula | C₂₀H₃₃NO₃S |
| Reversible | Y |
| Structure formula Image | |
| Applications |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 110110 | 0 |
Documentation
Operational Excellence Dossier
| Title |
|---|
N-Acetyl-S-farnesyl-L-cysteine Certificates of Analysis
| Title | Lot Number |
|---|---|
| 110110 |
References
| Reference overview |
|---|
| Molony, L., et al. 1996. Biochem. Biophys. Res. Commun. 223, 612. Ding, J., et al. 1994. J. Biol. Chem. 269, 16837. Philip, M.R., et al. 1993. Science 259, 977. Huzoor-Akbar, et al. 1991. J. Biol. Chem. 266, 4387. Perez-Sala, D., et al. 1991. Proc. Natl. Acad. Sci. USA 88, 3043. Volker, C., et al. 1991. J. Biol. Chem. 266, 21515. Hall, A. 1990. Science 249, 635. Yamane, H.K., et al. 1990. Proc. Natl. Acad. Sci. USA 87, 5868. |