444294 Sigma-AldrichMMP-2 Inhibitor IV - Calbiochem
The MMP-2 Inhibitor IV controls the biological activity of MMP-2. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
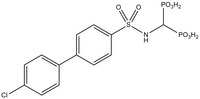
More>> The MMP-2 Inhibitor IV controls the biological activity of MMP-2. This small molecule/inhibitor is primarily used for Protease Inhibitors applications. Less<<Synonyms: Bone Resorption Inhibitor, (4'-chlorobiphenyl-4-ylsulfonamido)methylenediphosphonic acid
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₃H₁₄ClNO₈P₂S |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 444294-10MG | Glass bottle | 10 mg |
| Description | |
|---|---|
| Overview | A cell-permeable, bisphosphonate derivative that displays nanomolar activity against MMP-2 (IC50 = 37 nM), with good selectivity over MMP-8 (320 nM), MMP-9 (> 1 µM), and MMP-14 (> 1 µM). It is shown to inhibit osteoclast activity in a macrophage cell line, J774 (IC50 = 1.7 µM), which is more potent compared with other bisphosphonates such as Alendronate (Cat. No. 126855), (IC50 = 30 µM), and Zolendronate (7.8 µM), without observable cytotoxicity in HepG2 cells. At 25 µM, it abolishes the formation of actin rings, which are functional structures that are typical of resorbing osteoclasts, at activity levels comparable with those of zolendronic acid, inhibits bone resportion in vitro, and demonstrates cytotoxic properties in murine osteoclasts. |
| Catalogue Number | 444294 |
| Brand Family | Calbiochem® |
| Synonyms | Bone Resorption Inhibitor, (4'-chlorobiphenyl-4-ylsulfonamido)methylenediphosphonic acid |
| References | |
|---|---|
| References | Rubino, M.T., et al. 2011. Chem. Med. Chem. 6, 1258. |
| Product Information | |
|---|---|
| Form | Off-white powder |
| Hill Formula | C₁₃H₁₄ClNO₈P₂S |
| Chemical formula | C₁₃H₁₄ClNO₈P₂S |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 444294-10MG | 04055977186109 |
Documentation
MMP-2 Inhibitor IV - Calbiochem SDS
| Title |
|---|
MMP-2 Inhibitor IV - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 444294 |
References
| Reference overview |
|---|
| Rubino, M.T., et al. 2011. Chem. Med. Chem. 6, 1258. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|