401010 Sigma-AldrichI-BET - CAS 1260907-17-2 - Calbiochem
A cell-permeable benzodiazepine compound that binds the tandem bromodomains of BET (bromodomain and extra terminal domain) family members BRD2 (1-473), BRD3 (1-434), and BRD4 (1-477) with high affinity.
More>> A cell-permeable benzodiazepine compound that binds the tandem bromodomains of BET (bromodomain and extra terminal domain) family members BRD2 (1-473), BRD3 (1-434), and BRD4 (1-477) with high affinity. Less<<Synonyms: GSK525762A, (S)-2-(6-(4-Chlorophenyl)-8-methoxy-1-methyl-4H-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepin-4-yl)-N-ethylacetamide, (2-[(4chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-4-yl]-N-ethylacetamide, BRD2 Inhibitor I, BRD3 Inhibitor I, BRD4 Inhibitor I
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 1260907-17-2 | C₂₂H₂₂ClN₅O₂ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 401010-5MG | Glass bottle | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable benzodiazepine compound that binds the tandem bromodomains of BET (bromodomain and extra terminal domain) family members BRD2 (1-473), BRD3 (1-434), and BRD4 (1-477) with high affinity (Kd = 61.3, 50.5, and 55.2 nM, respectively, by ITC) and effectively competes against tetra-acetylated H4 peptide (Millipore cat. no. 12-379) for BRD2/3/4 binding (IC50 = 32.5, 42,4, and 36.1 nM, respectively, in competitive equilibrium binding assays), while exhibiting little affinity toward 5 other bromodomain-containing proteins (ATAD2, BAZ2B, CREBBP, PCAF, SP140) or a panel of 38 cellular enzymes, GPCRs, transporters, and ion channels. Shown to differentially modulate LPS-induced gene expression, notably suppressing LPS-induced upregulation of genes involved in inflammatory response, in murine BMDMs (bone marrow-derived macrophages) in vitro (30 min 1 µM pretreatment) and effectively prevent LPS-, heat-killed Salmonella typhimurium-, and CLP- (caecal ligation and puncture) induced death in mice in vivo (30 mg/kg i.v.). Cellular Brd2/3/4 knockdown using siRNA results in mostly the same effect as I-BET treatment in modulating LPS gene induction profile in murine BMDMs, but not without exceptions, indicating that not all BET-dependent transcription regulations are mediated via its binding to acetylated histones. Also available as a 50 mM solution in DMSO (Cat. No. 506071). |
| Catalogue Number | 401010 |
| Brand Family | Calbiochem® |
| Synonyms | GSK525762A, (S)-2-(6-(4-Chlorophenyl)-8-methoxy-1-methyl-4H-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepin-4-yl)-N-ethylacetamide, (2-[(4chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-4-yl]-N-ethylacetamide, BRD2 Inhibitor I, BRD3 Inhibitor I, BRD4 Inhibitor I |
| References | |
|---|---|
| References | Nicodeme, E., et al. 2010. Nature 468, 1119. |
| Product Information | |
|---|---|
| CAS number | 1260907-17-2 |
| Form | Yellowish white solid |
| Hill Formula | C₂₂H₂₂ClN₅O₂ |
| Chemical formula | C₂₂H₂₂ClN₅O₂ |
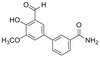
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by Chiral HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 401010-5MG | 04055977189391 |
Documentation
I-BET - CAS 1260907-17-2 - Calbiochem SDS
| Title |
|---|
I-BET - CAS 1260907-17-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 401010 |
References
| Reference overview |
|---|
| Nicodeme, E., et al. 2010. Nature 468, 1119. |