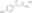346110 Sigma-AldrichGlucocorticoid Receptor Modulator, CpdA - CAS 14593-25-0 - Calbiochem
A cell-permeable aziridine precursor that acts as a nonsteroidal glucocorticoid receptor ligand with a binding affinity that is 4-fold higher than that of Dexamethasone.
More>> A cell-permeable aziridine precursor that acts as a nonsteroidal glucocorticoid receptor ligand with a binding affinity that is 4-fold higher than that of Dexamethasone. Less<<Synonyms: 2-((4-Acetoxyphenyl)-2-chloro-N-methyl)ethylammonium chloride
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 14593-25-0 | C₁₁H₁₄ClNO₂ • HCl |
| References | |
|---|---|
| References | De Bosscher, K., et al. 2005. Proc. Natl. Acad. Sci. USA 102, 15827. |
| Product Information | |
|---|---|
| CAS number | 14593-25-0 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₁H₁₄ClNO₂ • HCl |
| Chemical formula | C₁₁H₁₄ClNO₂ • HCl |
| Reversible | N |
| Structure formula Image | |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | A nonsteroidal glucocorticoid receptor (GR, Cat. No. 346100) ligand |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 346110 | 0 |
Documentation
Glucocorticoid Receptor Modulator, CpdA - CAS 14593-25-0 - Calbiochem SDS
| Title |
|---|
Glucocorticoid Receptor Modulator, CpdA - CAS 14593-25-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 346110 |
References
| Reference overview |
|---|
| De Bosscher, K., et al. 2005. Proc. Natl. Acad. Sci. USA 102, 15827. |