Interleukin-1beta and fibroblast growth factor receptor 1 cooperate to induce cyclooxygenase-2 during early mammary tumourigenesis.
Reed, Johanna R, et al.
Breast Cancer Res., 11: R21 (2009)
2009
Show Abstract
Inflammation within the tumour microenvironment correlates with increased invasiveness and poor prognosis in many types of cancer, including breast cancer. We have previously demonstrated that activation of a mouse mammary tumour virus (MMTV)-driven inducible fibroblast growth factor receptor 1 (iFGFR1) transgene in mammary epithelial cells results in an inflammatory response characterised by induction of inflammatory genes in the mammary gland. Specifically, we have observed increased levels of IL-1beta expression in the mammary gland following activation of iFGFR1 and have used the iFGFR1 model to elucidate the function of IL-1beta in promoting iFGFR1-induced mammary lesions. | 19393083
 |
Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration.
Runyan, C; Schaible, K; Molyneaux, K; Wang, Z; Levin, L; Wylie, C
Development (Cambridge, England)
133
4861-9
2006
Show Abstract
During germ-cell migration in the mouse, the dynamics of embryo growth cause many germ cells to be left outside the range of chemoattractive signals from the gonad. At E10.5, movie analysis has shown that germ cells remaining in the midline no longer migrate directionally towards the genital ridges, but instead rapidly fragment and disappear. Extragonadal germ cell tumors of infancy, one of the most common neonatal tumors, are thought to arise from midline germ cells that failed to die. This paper addresses the mechanism of midline germ cell death in the mouse. We show that at E10.5, the rate of apoptosis is nearly four-times higher in midline germ cells than those more laterally. Gene expression profiling of purified germ cells suggests this is caused by activation of the intrinsic apoptotic pathway. We then show that germ cell apoptosis in the midline is activated by down-regulation of Steel factor (kit ligand) expression in the midline between E9.5 and E10.5. This is confirmed by the fact that removal of the intrinsic pro-apoptotic protein Bax rescues the germ-cell apoptosis seen in Steel null embryos. Two interesting things are revealed by this: first, germ-cell proliferation does not take place in these embryos after E9.0; second, migration of germ cells is highly abnormal. These data show first that changing expression of Steel factor is required for normal midline germ cell death, and second, that Steel factor is required for normal proliferation and migration of germ cells. | 17107997
 |
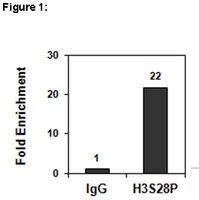
Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation.
Goto, H, et al.
J. Biol. Chem., 274: 25543-9 (1999)
1999
Show Abstract
Histone H3 (H3) phosphorylation at Ser(10) occurs during mitosis in eukaryotes and was recently shown to play an important role in chromosome condensation in Tetrahymena. When producing monoclonal antibodies that recognize glial fibrillary acidic protein phosphorylation at Thr(7), we obtained some monoclonal antibodies that cross-reacted with early mitotic chromosomes. They reacted with 15-kDa phosphoprotein specifically in mitotic cell lysate. With microsequencing, this phosphoprotein was proved to be H3. Mutational analysis revealed that they recognized H3 Ser(28) phosphorylation. Then we produced a monoclonal antibody, HTA28, using a phosphopeptide corresponding to phosphorylated H3 Ser(28). This antibody specifically recognized the phosphorylation of H3 Ser(28) but not that of glial fibrillary acidic protein Thr(7). Immunocytochemical studies with HTA28 revealed that Ser(28) phosphorylation occurred in chromosomes predominantly during early mitosis and coincided with the initiation of mitotic chromosome condensation. Biochemical analyses using (32)P-labeled mitotic cells also confirmed that H3 is phosphorylated at Ser(28) during early mitosis. In addition, we found that H3 is phosphorylated at Ser(28) as well as Ser(10) when premature chromosome condensation was induced in tsBN2 cells. These observations suggest that H3 phosphorylation at Ser(28), together with Ser(10), is a conserved event and is likely to be involved in mitotic chromosome condensation. | 10464286
 |
Increased phosphorylation of histone H1 in mouse fibroblasts transformed with oncogenes or constitutively active mitogen-activated protein kinase kinase.
Chadee, D N, et al.
J. Biol. Chem., 270: 20098-105 (1995)
1995
Show Abstract
We compared the nucleosomal organization, histone H1 subtypes, and histone H1 phosphorylated isoforms of ras-transformed and parental 10T1/2 mouse fibroblasts. In agreement with previous studies, we found that ras-transformed mouse fibroblasts have a less condensed chromatin structure than normal fibroblasts. ras-transformed and parental 10T1/2 cells had similar amounts of H1 subtypes, proteins that have a key role in the compaction of chromatin. However, labeling studies with 32P and Western blot experiments with an antiphosphorylated H1 antibody show that interphase ras-transformed cells have higher levels of phosphorylated H1 isoforms than parental cells. G1/S phase-arrested ras-transformed cells had higher amounts of phosphorylated H1 than G1/S phase-arrested parental cells. Mouse fibroblasts transformed with fes, mos, raf, myc, or constitutively active mitogen-activated protein (MAP) kinase kinase had increased levels of phosphorylated H1. These observations suggest that increased phosphorylation of H1 is one of the consequences of the persistent activation of the mitogen-activated protein kinase signal transduction pathway. Indirect immunofluorescent studies show that phosphorylated H1b is localized in centers of RNA splicing in the nucleus, suggesting that this modified H1 subtype is complexed to transcriptionally active chromatin. | 7650028
 |