181315 Sigma-AldrichARE Activator, BTZO-1 - Calbiochem
The ARE Activator, BTZO-1 modulates the biological activity of ARE. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The ARE Activator, BTZO-1 modulates the biological activity of ARE. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<Synonyms: 2-Pyridin-2-yl-4H-1,3-benzothiazin-4-one, Antioxidant Response Element Activator, Macrophage Migration Inhibitory Factor Antagonist II, MIF Antagonist II
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
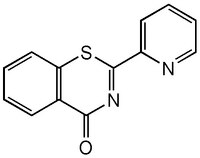
| C₁₃H₈N₂OS |
| Description | |
|---|---|
| Overview | This product has been discontinued. A cell-permeable benzothiazinone that is shown to interact with MIF homotrimer (Kd = 68.6 nM and 157 nM toward human and rat MIF, respectively, at pH 7.3) via the N-terminal proline in a pH-dependent manner, limiting its inhibitory capability against MIF tautomerase activity (13% inhibition at pH 6.0; [BTZO-1] = 30 µM) due to reduced MIF binding at acidic pH. Shown to protect primary rat cardiomyocytes (330 nM) from apoptotic death upon serum deprivation or DOX (200 nM; Cat. No. 324380) treatment via activation of ARE- (antioxidant response element) mediated cytoprotective genes transcription in a MIF-dependent manner. |
| Catalogue Number | 181315 |
| Brand Family | Calbiochem® |
| Synonyms | 2-Pyridin-2-yl-4H-1,3-benzothiazin-4-one, Antioxidant Response Element Activator, Macrophage Migration Inhibitory Factor Antagonist II, MIF Antagonist II |
| References | |
|---|---|
| References | Kimura, H., et al. 2010. Chem. Biol. 17, 1282. |
| Product Information | |
|---|---|
| Form | Off-white solid |
| Hill Formula | C₁₃H₈N₂OS |
| Chemical formula | C₁₃H₈N₂OS |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 181315 | 0 |
Documentation
ARE Activator, BTZO-1 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Kimura, H., et al. 2010. Chem. Biol. 17, 1282. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|