262017 Sigma-AldrichAPE1 Inhibitor III - CAS 524708-03-0 - Calbiochem
The APE1 Inhibitor III controls the biological activity of APE1. This small molecule/inhibitor is primarily used for Cell Structure applications.
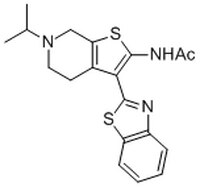
More>> The APE1 Inhibitor III controls the biological activity of APE1. This small molecule/inhibitor is primarily used for Cell Structure applications. Less<<Synonyms: N-(3-(1,3-Benzo[d]thiazol-2-yl)-6-isopropyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridin-2-yl)acetamide, Apurinic Endonuclease 1 Inhibitor III, Apurinic/Apyrimidinic Endonuclease 1 Inhibitor III
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 524708-03-0 | C₁₉H₂₁N₃OS₂ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 262017-10MG | 10 mg |
| References | |
|---|---|
| References | Rai, G., et al. 2012. J. Med. Chem. 55, 3101. |
| Product Information | |
|---|---|
| CAS number | 524708-03-0 |
| Form | Brown solid |
| Hill Formula | C₁₉H₂₁N₃OS₂ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | APE1 |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 262017-10MG | 04055977216981 |
Documentation
APE1 Inhibitor III - CAS 524708-03-0 - Calbiochem SDS
| Title |
|---|
APE1 Inhibitor III - CAS 524708-03-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 262017 |
References
| Reference overview |
|---|
| Rai, G., et al. 2012. J. Med. Chem. 55, 3101. |