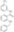676504 Sigma-AldrichVEGFR Tyrosine Kinase Inhibitor VI, AAL-993 - CAS 269390-77-4 - Calbiochem
The VEGFR Tyrosine Kinse Inhibitor VI, ALL-993, also referenced under CAS 269390-77-4, controls the biological activity of VEGFR Tyrosine Kinase. This small molecule/inhibitor is primarily used for Cancer applications.
More>> The VEGFR Tyrosine Kinse Inhibitor VI, ALL-993, also referenced under CAS 269390-77-4, controls the biological activity of VEGFR Tyrosine Kinase. This small molecule/inhibitor is primarily used for Cancer applications. Less<<Synonyms: 2-((4-Pyridyl)methyl)amino-N-(3-(trifluoromethyl)phenyl)benzamide, ZK260253, PDGFR Tyrosine Kinase Inhibitor XXIII, VEGFR2 Kinase Inhibitor XXXIII, VEGFR1 Kinase Inhibitor VIII, VEGFR3 Kinase Inhibitor VII, AAL993
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 269390-77-4 | C₂₀H₁₆F₃N₃O |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 676504-10MG | Glass bottle | 10 mg |
| Description | |
|---|---|
| Overview | A cell-permeable anthranilamide that acts as a potent VEGFR inhibitor (IC50 = 130, 23, and 18 nM against VEGFR-1, -2, and -3, respectively; IC50 = 1.24 nM against VEGF-induced human VEGFR-2 phosphorylation in CHO cells) by targeting the ATP-binding site of VEGFR in its inactive "DFG-out" conformation and effectively suppresses tumor growths (50 to 100 mg/kg; p.o.) via its anti-angiogenesis activity in mice and rats in vivo, while inhibiting c-kit, CSF-1R/c-Fms, PDGFR-β, and c-Abl only at higher concentrations (IC50 = 236, 380, 640, and 2820 nM, respectively). AAL933 and two other VEGFR inhibitors, KRN633 and SU5416 (Cat. No. 676487), are also shown to inhibit hypoxia-induced HIF-1α expression (by <90% at 30 µM) and transcription activation. Unlike KRN633 and SU5416, AAL933 prevents only hypoxia-induced Erk, but not Akt, phosphorylation in HeLa cells. |
| Catalogue Number | 676504 |
| Brand Family | Calbiochem® |
| Synonyms | 2-((4-Pyridyl)methyl)amino-N-(3-(trifluoromethyl)phenyl)benzamide, ZK260253, PDGFR Tyrosine Kinase Inhibitor XXIII, VEGFR2 Kinase Inhibitor XXXIII, VEGFR1 Kinase Inhibitor VIII, VEGFR3 Kinase Inhibitor VII, AAL993 |
| References | |
|---|---|
| References | Ban, H.S., et al. 2010. Cancer Lett. 296, 17; Manley, P.W., et al. 2004. Biochim. Biophys. Acta 1697, 17; Manley, P.W., et al. 2002. J. Med. Chem. 45, 5687. |
| Product Information | |
|---|---|
| CAS number | 269390-77-4 |
| Form | Off-white powder |
| Hill Formula | C₂₀H₁₆F₃N₃O |
| Chemical formula | C₂₀H₁₆F₃N₃O |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 676504-10MG | 04055977260533 |
Documentation
VEGFR Tyrosine Kinase Inhibitor VI, AAL-993 - CAS 269390-77-4 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Ban, H.S., et al. 2010. Cancer Lett. 296, 17; Manley, P.W., et al. 2004. Biochim. Biophys. Acta 1697, 17; Manley, P.W., et al. 2002. J. Med. Chem. 45, 5687. |
Brochure
| Title |
|---|
| New Products: Volume 3, 2012 |
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|