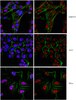
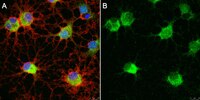
Effects of the transcription factor Olig1 on the differentiation and remyelination of oligodendrocyte precursor cells after focal cerebral ischemia in rats
Hong Zhao 1 , Xiao-Yu Gao 2 , Zan-Hua Liu 3 , Jian-Wen Lin 1 , Su-Ping Wang 1 , De-Xin Wang 4 , Yong-Bo Zhang
Mol Med Rep
20(5)
4603-4611
2019
Show Abstract
The differentiation and maturation of oligodendrocyte precursor cells (OPCs) is important for remyelination in the central nervous system. Nevertheless, this process is often limited and incomplete in ischemic injury. Oligodendrocyte transcription factor 1 (Olig1) is important for the maturation of OPCs and the repair of demyelinated lesions. However, how Olig1 modulates the development of OPCs or the remyelination associated with ischemic injury remains unclear. The present study aimed to examine alterations in OPCs, and the expression of myelin and Olig1, at different time-points after focal cerebral ischemia using immunohistochemistry and western blot techniques to elucidate the role of Olig1 in the maturation of OPCs and remyelination. The present results showed that the expression of Olig1 significantly decreased at 1 day after middle cerebral artery occlusion (MCAO) and returned to normal levels from day 3 to 28. Additionally, Olig1 was found to translocate into the nucleus following ischemia in the brain. The number of OPCs in the ischemic striatum significantly declined at days 1 and 3 following MCAO, and increased at days 7, 14 and 28 compared with the control. The expression of myelin basic protein, a marker of mature oligodendrocytes and myelin, gradually decreased from day 1 to 7 after ischemia and recovered at day 14 and 28; however, the levels were lower than those in the control group. The present results indicated that the restored normal level of Olig1 following ischemia may play an important role in the maturation of OPCs through its translocation into the nucleus, where it may promote the growth and development of myelin under pathological conditions. However, this endogenous recovery mechanism fails to fully repair the demyelinated lesion. The data of the present study may help clinicians understand the expression pattern of Olig1 and its potential role in endogenous remyelination after ischemia, which may have implications for the treatment of diseases that lead to demyelination. | 31702031
 |
NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis
Shu-Zhen Zhang 1 , Qin-Qin Wang 1 2 3 , Qiao-Qiao Yang 1 , Huan-Yu Gu 4 , Yan-Qing Yin 1 , Yan-Dong Li 1 , Jin-Can Hou 1 , Rong Chen 1 2 , Qing-Qing Sun 1 2 , Ying-Feng Sun 5 , Gang Hu 4 , Jia-Wei Zhou
BMC Med
17(1)
204
2019
Show Abstract
Background: Brain innate immunity is vital for maintaining normal brain functions. Immune homeostatic imbalances play pivotal roles in the pathogenesis of neurological diseases including Parkinson's disease (PD). However, the molecular and cellular mechanisms underlying the regulation of brain innate immunity and their significance in PD pathogenesis are still largely unknown. <br />Methods: Cre-inducible diphtheria toxin receptor (iDTR) and diphtheria toxin-mediated cell ablation was performed to investigate the impact of neuron-glial antigen 2 (NG2) glia on the brain innate immunity. RNA sequencing analysis was carried out to identify differentially expressed genes in mouse brain with ablated NG2 glia and lipopolysaccharide (LPS) challenge. Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice were used to evaluate neuroinflammatory response in the presence or absence of NG2 glia. The survival of dopaminergic neurons or glial cell activation was evaluated by immunohistochemistry. Co-cultures of NG2 glia and microglia were used to examine the influence of NG2 glia to microglial activation. <br />Results: We show that NG2 glia are required for the maintenance of immune homeostasis in the brain via transforming growth factor-β2 (TGF-β2)-TGF-β type II receptor (TGFBR2)-CX3C chemokine receptor 1 (CX3CR1) signaling, which suppresses the activation of microglia. We demonstrate that mice with ablated NG2 glia display a profound downregulation of the expression of microglia-specific signature genes and remarkable inflammatory response in the brain following exposure to endotoxin lipopolysaccharides. Gain- or loss-of-function studies show that NG2 glia-derived TGF-β2 and its receptor TGFBR2 in microglia are key regulators of the CX3CR1-modulated immune response. Furthermore, deficiency of NG2 glia contributes to neuroinflammation and nigral dopaminergic neuron loss in MPTP-induced mouse PD model. <br />Conclusions: These findings suggest that NG2 glia play a critical role in modulation of neuroinflammation and provide a compelling rationale for the development of new therapeutics for neurological disorders. | 31727112
 |
Self-organized developmental patterning and differentiation in cerebral organoids
Magdalena Renner 1 , Madeline A Lancaster 1 2 , Shan Bian 1 , Heejin Choi 3 , Taeyun Ku 3 , Angela Peer 1 , Kwanghun Chung 3 4 5 6 7 , Juergen A Knoblich
EMBO J
36(10)
1316-1329
2017
Show Abstract
Cerebral organoids recapitulate human brain development at a considerable level of detail, even in the absence of externally added signaling factors. The patterning events driving this self-organization are currently unknown. Here, we examine the developmental and differentiative capacity of cerebral organoids. Focusing on forebrain regions, we demonstrate the presence of a variety of discrete ventral and dorsal regions. Clearing and subsequent 3D reconstruction of entire organoids reveal that many of these regions are interconnected, suggesting that the entire range of dorso-ventral identities can be generated within continuous neuroepithelia. Consistent with this, we demonstrate the presence of forebrain organizing centers that express secreted growth factors, which may be involved in dorso-ventral patterning within organoids. Furthermore, we demonstrate the timed generation of neurons with mature morphologies, as well as the subsequent generation of astrocytes and oligodendrocytes. Our work provides the methodology and quality criteria for phenotypic analysis of brain organoids and shows that the spatial and temporal patterning events governing human brain development can be recapitulated in vitro. | 28283582
 |















 Antibody[201874-ALL].jpg)