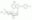116812 Sigma-AldrichAdenosine 3ʹ,5ʹ-cyclic Monophosphate, 8-(4-Chlorophenylthio)-, Sodium Salt - CAS 93882-12-3 - Calbiochem
Cell-permeable cAMP analog that activates both protein kinase A and protein kinase G.
More>> Cell-permeable cAMP analog that activates both protein kinase A and protein kinase G. Less<<Synonyms: Adenosine 3ʹ,5ʹ-cyclic Monophosphate, 8-(4-Chlorophenylthio)-, Sodium Salt
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 93882-12-3 | C₁₆H₁₄ClN₅O₆PS · Na |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 116812-10MG | Plastic ampoule | 10 mg |
| Product Information | |
|---|---|
| CAS number | 93882-12-3 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₆H₁₄ClN₅O₆PS · Na |
| Chemical formula | C₁₆H₁₄ClN₅O₆PS · Na |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Protein kinase A and protein kinase G |
| Primary Target IC<sub>50</sub> | 900 nM against cGMP-specific phosphodiesterase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 116812-10MG | 04055977208238 |
Documentation
Adenosine 3ʹ,5ʹ-cyclic Monophosphate, 8-(4-Chlorophenylthio)-, Sodium Salt - CAS 93882-12-3 - Calbiochem SDS
| Title |
|---|
Adenosine 3ʹ,5ʹ-cyclic Monophosphate, 8-(4-Chlorophenylthio)-, Sodium Salt - CAS 93882-12-3 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 116812 |
References
| Reference overview |
|---|
| de Rooij, J., et al. 1998. Nature. 396, 474. Kawasaki, H., et al. 1998. Science. 282, 2275. Moed, P.J., et al. 1993. Comp. Biochem. Physiol. 105A, 711. Connolly, B.J., et al. 1992. Biochem. Pharmacol. 44, 2303. Phillips, M.E., and Taylor, A. 1992. J. Physiol. 456, 591. Shipston, M.J., and Antoni, F.A. 1992. Endocrinology 130, 2213. Datta, R., et al. 1991. Blood 78, 83. Peters, D.J.M., et al. 1991. Proc. Natl. Acad. Sci. USA 88, 9219. Sandberg, M., et al. 1991. Biochem. J. 279, 521. Unterman, T.G., et al. 1991. Endocrinology 128, 2693. Xu, H., et al. 1989. Brain Res. 504, 36. |