QIA10 Sigma-Aldrichc-ErbB2/c-Neu Rapid Format ELISA Kit
Recommended Products
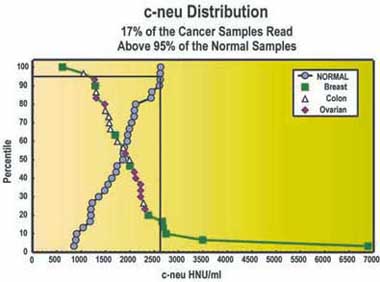
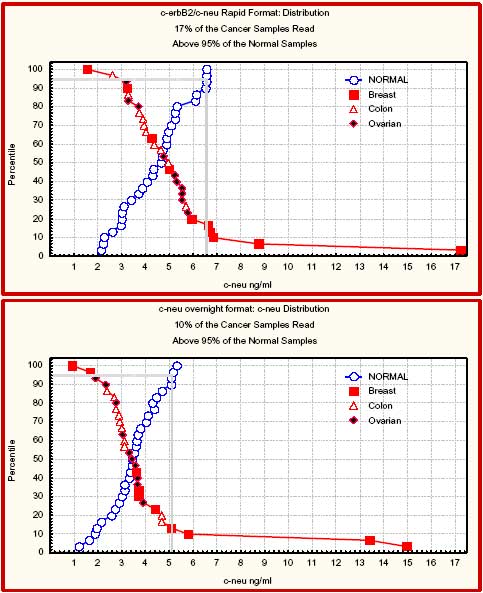
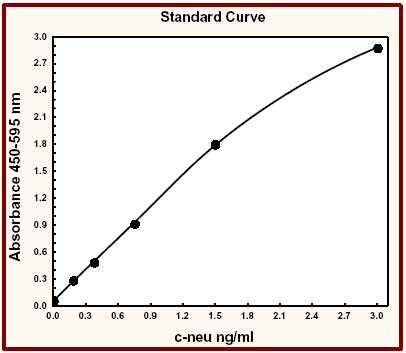
Overview
| Replacement Information |
|---|
Key Spec Table
| Species Reactivity |
|---|
| H |
| Applications |
|---|
| Biological Information | |
|---|---|
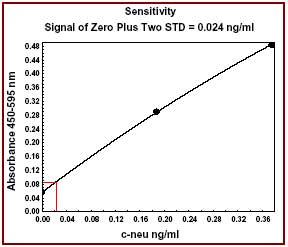
| Assay range | 0-3 ng/ml |
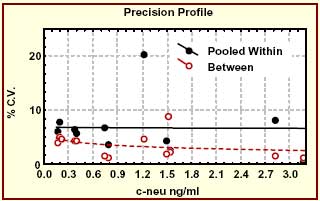
| Assay time | 4 h |
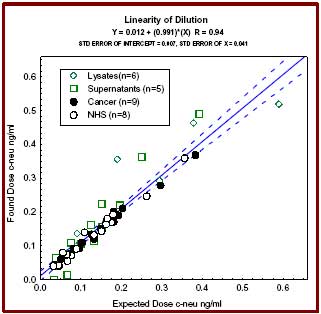
| Sample Type | Cell lysates, biological fluids |
| Species Reactivity |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| QIA10 | 0 |
Documentation
c-ErbB2/c-Neu Rapid Format ELISA Kit SDS
| Title |
|---|
c-ErbB2/c-Neu Rapid Format ELISA Kit Certificates of Analysis
| Title | Lot Number |
|---|---|
| QIA10 |
References
| Reference overview |
|---|
| Gauchez, A.S., et al. 2008. Anticancer Res. 28, 3067. Hung, M.-C. and Y-K Lau, 1999. Seminars in Oncology 26(Suppl 12), 51. Ouyang, X., et al. 1999. Lancet 353, 1591. Ross, J.S. and J.A. Fletcher, 1999. Am. J. Clin. Pathol. 112, S53. Ross, J.S. and J.A. Fletcher, 1998. Stem Cells 16, 413. Disis, M.L., et al. 1997. J. Clin. Oncol. 15, 3363. Ross, J.S., et al. 1997. Human Pathology 28, 827. Ross, J.S., et al. 1997. Cancer 79, 2162. Xia, W., et al. 1997. Clin. Cancer Res. 3, 3. Cirisano, F.D. and B.Y. Karlan, 1996. J. Soc. Gynecol. Invest. 3, 99. Menden, H., et al. 1994. Cancer Res. Clin. Oncol. 120, 378. Kynast, B., et al. 1993. J. Cancer Res. Clin. Oncol. 119, 249. Gusterson, B. and R. Gelber, 1992. J. Clin. Oncol. 10, 1049. Hetzel, D.J., et al. 1992. Gynecol. Oncol. 47, 179. Hou, L., et al. 1992. Cancer Lett. 65, 215. Leitzel, K., et al. 1992. J. Clin. Oncol. 10, 1436. Carney, W.P., et al. 1991. J. Tumor Marker Oncol. 6, p. 53. Clark, G.M. and W.L. McGuire, 1991. Cancer Res. 51, 944. Langton, B.C., et al. 1991. Cancer Res. 51, 2593. Paterson, M.C., et al. 1991. Cancer Res. 51, 556. Zabrecky, J.R., et al .1991. J. Biol. Chem. 266, 1716. Berchuck, A., et al. 1990. Cancer Res. 50, 4087. Borg, A., et. al. 1990. Cancer Res. 50, 4332. Inglehart, J.D., et al. 1990. Cancer Res. 50, 6701. Kern, J.A., et al. 1990. Cancer Res. 50, 5189. Mori, S., et al. 1990. Jpn. J. Cancer Res. 81, 489. Paik, S., et al. 1990. J. Clin. Oncol. 8, 103. Maguire, H.C. and M.I. Greene, 1989. Seminars in Oncology 16, 148. Ro, J., et al. 1989. Cancer Res. 49, 6941. Slamon, D.J., et al. 1989. Science 244, 707. Tandon, A.K., et al. 1989. J. Clin. Oncol. 7, 1120. Wright, C., et al. 1989. Cancer Res. 49, 2087. Yoshida, K., et al. 1989. Virchows Arch. B Cell Pathol. Mol. Pathol. 57, 285. Fontaine, J., et al. 1988. Oncology 45, 360. Kraus, M.H., et al., 1987. EMBO J. 6, 605. Slamon, D., et al. 1987. Science 235, 177. King, C.R., et al. 1985. Science 229, 974. Shin, C., et al. 1981. Nature 290, 261. Henry, R.J., et al. 1974. Clinical Chemistry, Harper and Row, New York. |
Brochure
| Title |
|---|
| Bulk Product Guide |
| Protein Kinase Assay and Detection Kits Brochure |