540222 Sigma-AldrichPuromycin, Dihydrochloride - CAS 58-58-2 - Calbiochem
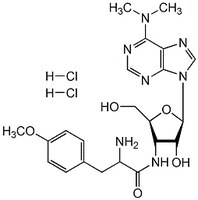
Puromycin, Dihydrochloride, CAS 58-58-2, is An aminonucleoside antibiotic that inhibits protein synthesis by blocking the translation step and causes premature release of nascent polypeptide chains.
More>> Puromycin, Dihydrochloride, CAS 58-58-2, is An aminonucleoside antibiotic that inhibits protein synthesis by blocking the translation step and causes premature release of nascent polypeptide chains. Less<<Synonyms: 3ʹ-(α-Amino-p-methoxyhydrocinnamamido)-3ʹ-deoxy-N,N-dimethyladenosine, 2HCl
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 58-58-2 | C₂₂H₂₉N₇O₅ · 2HCl |
| Product Information | |
|---|---|
| CAS number | 58-58-2 |
| Form | White to off-white solid |
| Hill Formula | C₂₂H₂₉N₇O₅ · 2HCl |
| Chemical formula | C₂₂H₂₉N₇O₅ · 2HCl |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | protein synthesis |
| Purity | ≥98% by TLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | AU7355000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 540222 | 0 |
Documentation
Puromycin, Dihydrochloride - CAS 58-58-2 - Calbiochem SDS
| Title |
|---|
Puromycin, Dihydrochloride - CAS 58-58-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 540222 |
References
| Reference overview |
|---|
| Chow, S.C., et al. 1995. Exp. Cell Res. 216, 149. Constam, D.B., et al. 1995. J. Biol. Chem. 270, 26931. Wirth, M., et al. 1994. J. Virol. 68, 566. Claeyssens, S., et al. 1993. FEBS Lett. 315, 7. Hechler, U., et al. 1993. Biochem. Biophys. Res. Commun. 194, 1305. Kaufman, S.H., et al. 1993. Cancer Res. 53, 3976. de la Luna, S. and Ortín, J. 1992. Methods Enzymol. 216, 376. Kalpaxis, D.L. and Drainas, D. 1992. Arch. Biochem. Biophys. 300, 629. Shipston, M.J. and Antoni, F.A. 1992. Biochem. Biophys. Res. Commun. 189, 1382. |
Brochure
| Title |
|---|
| Antibiotics Profiler |
| Caspases and other Apoptosis Related Tools Brochure |