475889 Sigma-AldrichMK-886 - CAS 118414-82-7 - Calbiochem
A cell-permeable, orally active NSAID that blocks cellular Cox pathway PGE2 production by inhibiting COX-1 and mPGES-1, but not COX-2, activity, as well as suppresses cellular 5-LO pathway activation by inhibiting FLAP, rather than 5-LO, activity.
More>> A cell-permeable, orally active NSAID that blocks cellular Cox pathway PGE2 production by inhibiting COX-1 and mPGES-1, but not COX-2, activity, as well as suppresses cellular 5-LO pathway activation by inhibiting FLAP, rather than 5-LO, activity. Less<<Sinónimos: 3-[1-(p-Chlorobenzyl)-5-(isopropyl)-3-t-butylthioindol-2-yl]-2,2-dimethylpropanoic Acid, Na, COX-1 Inhibitor III, FLAP Inhibitor I, MK886, mPGES-1 Inhibitor I
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
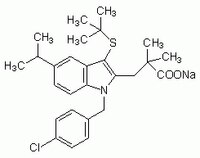
| 118414-82-7 | C₂₇H₃₃ClNO₂S · Na |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 475889-5MG |
|
Ampolla de plást. | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable, orally active NSAID (nonsteroidal antiinflammatory drug) that blocks cellular Cox pathway PGE2 (prostaglandin E2) production by inhibiting COX-1 and mPGES-1 (microsomal PGE2 synthase-1), but not COX-2, activity (IC50 = 8, 2, and 58 µM, respectively), as well as suppresses cellular 5-LO (5-Lypoxygenase; Cat. No. 437996) pathway activation by inhibiting FLAP (5-LO-activating protein), rather than 5-LO, activity (<10% by 1 µM MK-886). Unlike NSAIDs (nonsteroidal antiinflammatory drugs) that target only COX pathway, MK-886 does not cause gastrointestinal damages when applied in vivo. |
| Catalogue Number | 475889 |
| Brand Family | Calbiochem® |
| Synonyms | 3-[1-(p-Chlorobenzyl)-5-(isopropyl)-3-t-butylthioindol-2-yl]-2,2-dimethylpropanoic Acid, Na, COX-1 Inhibitor III, FLAP Inhibitor I, MK886, mPGES-1 Inhibitor I |
| Product Information | |
|---|---|
| CAS number | 118414-82-7 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₇H₃₃ClNO₂S · Na |
| Chemical formula | C₂₇H₃₃ClNO₂S · Na |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | leukotreine biosybthesis |
| Primary Target IC<sub>50</sub> | 102 nM |
| Purity | ≥99% by TLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 475889-5MG | 04055977203592 |
Documentation
MK-886 - CAS 118414-82-7 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
MK-886 - CAS 118414-82-7 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 475889 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Koeberle, A., et al. 2009. Eur. J. Pharmacol. 608, 84. Koeberle, A., et al. 2008. J. Pharmacol. Exp. Ther. 326, 975. Fisher, L., et al. 2007. Br. J. Pharmacol. 152, 471. Ford-Hutchinson, A.W., et al. 1993. Can. J. Physiol. Pharmacol. 71, 806. Ford-Hutchinson, A.W. 1991. Trends Pharmacol. 12, 68. Dixon, R.A., et al. 1990. Nature 343, 282. Rouzer, C.A., et al. 1990. J. Biol. Chem. 265, 1436. |
| Ficha técnica | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|