444967 Sigma-AldrichMEK1/2 Inhibitor IV - CAS 212631-67-9 - Calbiochem
The MEK1/2 Inhibitor IV, also referenced under CAS 212631-67-9, controls the biological activity of MEK1/2. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The MEK1/2 Inhibitor IV, also referenced under CAS 212631-67-9, controls the biological activity of MEK1/2. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<Sinónimos: 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-5-bromo-benzamide, PD184161, MEK Inhibitor IV
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
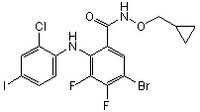
| 212631-67-9 | C₁₇H₁₃BrClF₂IN₂O₂ |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 444967-5MG |
|
5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable, blood-brain barrier permeant, and orally active hydroxamate compound that is reported to inhibit MEK activity (IC50 = 10-100 nM) without competing against ATP or Erk binding and exhibit excellent selectivity over 27 other cellular kinases, including JNK, MAPK2/ERK2, SAPK2a, SAPK2b, SAPK3, and SAPK4 (IC50 > 10 µM). Shown to be superior to PD 98059 (Cat. Nos. 513000 and 513001) and U0126 (Cat. No. 662005) in suppressing Erk1/2 phosphorylation in Hep3B, HepG2, PLC, and SKHep human liver cancer cells (IC50 <0.1 µM) in vitro and effectively reduce Erk1/2 phosphorylation in hippocampal tissue in mice (ED50 <50 mg/kg, i.p.) in vivo. Both PD184161 and U0126 are shown to induce necrosis of several types of glucose-deprived cells via an indirect action on the F0 component of the mitochondrial F1F0-ATPase/synthase. |
| Catalogue Number | 444967 |
| Brand Family | Calbiochem® |
| Synonyms | 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-5-bromo-benzamide, PD184161, MEK Inhibitor IV |
| Product Information | |
|---|---|
| CAS number | 212631-67-9 |
| Form | White solid |
| Hill Formula | C₁₇H₁₃BrClF₂IN₂O₂ |
| Chemical formula | C₁₇H₁₃BrClF₂IN₂O₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 444967-5MG | 04055977204537 |
Documentation
MEK1/2 Inhibitor IV - CAS 212631-67-9 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
MEK1/2 Inhibitor IV - CAS 212631-67-9 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 444967 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Yip-Schneider, M.T., et al. 2009. J. Pharmacol. Exp. Ther. 329, 1063. Duman, C.H., et al. 2007. Biol. Psychiatry 61, 661. Klein, P.J., et al. 2006. Neoplasia 8, 1. Thottassery, J.V., et al. 2004. Cancer Res. 64, 4637. Yung, H.W., et al. 2004. Biochem. Pharmacol. 68, 351. |