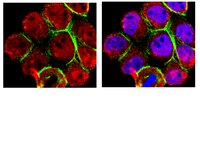
Lack of synergistic effect of resveratrol and sigma-1 receptor agonist (PRE-084) in SOD1G⁹³A ALS mice: overlapping effects or limited therapeutic opportunity?
Mancuso, R; Del Valle, J; Morell, M; Pallás, M; Osta, R; Navarro, X
Orphanet journal of rare diseases
9
78
2014
Mostrar resumen
Amyotrophic lateral sclerosis (ALS) is an adult onset neurodegenerative disease characterized by the loss of motoneurons (MNs) in the spinal cord, brainstem and motor cortex, causing progressive paralysis and death. Nowadays, there is no effective therapy and most patients die 2-5 years after diagnosis. Sigma-1R is a transmembrane protein highly expressed in the CNS and specially enriched in MNs. Mutations on the Sigma-1R leading to frontotemporal lobar degeneration-ALS were recently described in human patients. We previously reported the therapeutic role of the selective sigma-1R agonist 2-(4-morpholi-nethyl)1-phenylcyclohexanecarboxylate (PRE-084) in SOD1G93A ALS mice, that promoted spinal MN preservation and extended animal survival by controlling NMDA receptor calcium influx. Resveratrol (RSV, trans-3,4',5-trihydroxystilbene) is a natural polyphenol with promising neuroprotective effects. We recently found that RSV administration to SOD1G93A mice preserves spinal MN function and increases mice survival. These beneficial effects were associated to activation of Sirtuin 1 (Sirt1) and AMP-activated protein kinase (AMPK) pathways, leading to the modulation of autophagy and an increase of mitochondrial biogenesis. The main goal of this work was to assess the effect of combined RSV and PRE-084 administration in SOD1G93A ALS mice.We determined the locomotor performance of the animals by rotarod test and evaluated spinal motoneuron function using electrophysiological tests.RSV plus PRE-084 treatment from 8 weeks of age significantly improved locomotor performance and spinal MN function, accompanied by a significant reduction of MN degeneration and an extension of mice lifespan. In agreement with our previous findings, there was an induction of PKC-specific phosphorylation of the NMDA-NR1 subunit and an increased expression and activation of Sirt1 and AMPK in the ventral spinal cord of treated SOD1G93A animals.Although combined PRE and RSV treatment significantly ameliorated SOD1G93A mice, it did not show a synergistic effect compared to RSV-only and PRE-084-only treated groups. | Western Blotting | 24885036
 |
Exercise Increases Markers of Spermatogenesis in Rats Selectively Bred for Low Running Capacity.
Torma, F; Koltai, E; Nagy, E; Ziaaldini, MM; Posa, A; Koch, LG; Britton, SL; Boldogh, I; Radak, Z
PloS one
9
e114075
2014
Mostrar resumen
The oxidative stress effect of exercise training on testis function is under debate. In the present study we used a unique rat model system developed by artificial selection for low and high intrinsic running capacity (LCR and HCR, respectively) to evaluate the effects of exercise training on apoptosis and spermatogenesis in testis. Twenty-four 13-month-old male rats were assigned to four groups: control LCR (LCR-C), trained LCR (LCR-T), control HCR (HCR-C), and trained HCR (HCR-T). Ten key proteins connecting aerobic exercise capacity and general testes function were assessed, including those that are vital for mitochondrial biogenesis. The VO2 max of LCR-C group was about 30% lower than that of HCR-C rats, and the SIRT1 levels were also significantly lower than HCR-C. Twelve weeks of training significantly increased maximal oxygen consumption in LCR by nearly 40% whereas HCR remained unchanged. LCR-T had significantly higher levels of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1α), decreased levels of reactive oxygen species and increased acetylated p53 compared to LCR-C, while training produced no significant changes for these measures in HCR rats. BAX and Blc-2 were not different among all four groups. The levels of outer dense fibers -1 (Odf-1), a marker of spermatogenesis, increased in LCR-T rats, but decreased in HCR-TR rats. Moreover, exercise training increased the levels of lactate dehydrogenase C (LDHC) only in LCR rats. These data suggest that rats with low inborn exercise capacity can increase whole body oxygen consumption and running exercise capacity with endurance training and, in turn, increase spermatogenesis function via reduction in ROS and heightened activity of p53 in testes. | Western Blotting | 25493948
 |
Vorinostat(SAHA) Promotes Hyper-Radiosensitivity in Wild Type p53 Human Glioblastoma Cells.
Diss, E; Nalabothula, N; Nguyen, D; Chang, E; Kwok, Y; Carrier, F
Journal of clinical oncology and research
2
2014
Mostrar resumen
Glioblastoma multiforme (GBM) is a very aggressive and locally invasive tumor. The current standard of care is partial brain radiation therapy (60 Gy) concurrently with the alkylating agent temozolomide (TMZ). However, patients' survival remains poor (6-12 months) mainly due to local and diffuse (distant) recurrence. The possibility to promote hyper radiosensitivity (HRS) with low dose radiation may contribute to improve outcome. Here, we evaluated the effect of Vorinostat(SAHA) and TMZ on glioblastoma cells' sensitivity to low dose radiation. Clonogenic survivals were performed on D54 (p53 and PTEN wild type) and U118 (p53 and PTEN mutants) cells exposed to clinically relevant doses of Vorinostat(SAHA) and TMZ and increasing radiation doses. Apoptosis was measured by the activation of caspase-3 and the role of p53 and PTEN were evaluated with the p53 inhibitor pifithrin α and the PI3K/AKT pathway inhibitor LY29002. Vorinostat(SAHA) promoted HRS at doses as low as 0.25 Gy in the D54 but not the U118 cells. Killing efficiency was associated with caspase-3 activation, delayed H2AX phosphorylation and abrogation of a radiation -induced G2 arrest. Inhibiting p53 function with pifithrin α prevented the promotion of HRS by Vorinostat(SAHA). Moreover, LY29002, a PI-3K inhibitor, restored promotion of HRS by Vorinostat(SAHA) in the p53 mutant U118 cells to levels similar to the p53 wild type cells. TMZ also promoted HRS at doses as low as 0.15 Gy. These finding indicate that HRS can be promoted in p53 wild type glioblastoma cells through a functional PTEN to delay DNA repair and sensitize cells to low dose radiation. Promotion of HRS thus appears to be a viable approach for GBM that could be used as a basis to develop new Phase I/II studies. | | 25379568
 |
ING5 is a Tip60 cofactor that acetylates p53 in response to DNA damage.
Liu, N; Wang, J; Wang, J; Wang, R; Liu, Z; Yu, Y; Lu, H
Cancer research
73
3749-60
2013
Mostrar resumen
Posttranslational modification of p53 is a critical event in regulating the expression of its target genes. p53 is acetylated at lysine 120 (K120) by acetyltranferases Tip60 (KAT5) and hMOF (KAT8) in response to DNA damage. Identification of cofactors for these two enzymes will shed light on the mechanism by which cells make a choice between cell-cycle arrest and apoptosis. It has been reported that ING5, a member of the inhibitor of growth (ING) family, is involved in p53-dependent pathways, but its exact role is unknown. In this study, we found that ING5 expression was significantly increased and that ING5 assisted Tip60, but not hMOF, in acetylating p53 at K120 in response to DNA damage. ING5 had no effect on acetylation of p53 at K373/382, but it formed a complex with p53 and Tip60. ING5 was required for acetylation of p53 at K120, and p53 acetylated at K120 subsequently bound to the promoters of its target apoptotic genes, BAX and GADD45, to promote their expression and lead to apoptosis. Mutation of K120 to K120R abolished the effects of ING5 on p53-induced gene expression. Thus, we conclude that ING5 functions as a cofactor of Tip60 in the acetylation of p53 at K120 in response to DNA damage. | | 23576563
 |
Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells.
Bell-Temin, Harris, et al.
J. Proteome Res., 12: 2067-77 (2013)
2013
Mostrar resumen
Long-term exposure to alcohol can have profound effects on the central nervous system including pathophysiological consequences associated with neuroinflammation. Along with astroglia, microglia play an important role in the neuroinflammatory response. Using a SILAC-labeled rat microglial cell line, an expression profile of 2994 proteins was identified in ethanol-treated microglial cells, where 160 and 69 protein groups were determined to be significantly upregulated and downregulated, respectively. In addition, SILAC-based proteomic analysis of lipopolysaccharide-treated microglial cells was performed in order to generate a reference data set representing a "classical" (M1) macrophage activation response in order to compare to the differential protein expression profile of ethanol-treated microglia. On the basis of this comparison as well as other validation experiments performed in this study, ethanol appears to induce partial activation of microglia that is devoid of conventional markers that indicate an M1 phenotype. This study is the first comprehensive proteomic analysis to assess the impact of acute ethanol exposure on microglial function and will provide a significant foundation that includes novel protein markers for future work aimed to characterize the molecular mechanisms associated with ethanol-induced microglial activation and its role in neurodegeneration. | | 23495833
 |
Histone deacetylase inhibitors enhance the anticancer activity of nutlin-3 and induce p53 hyperacetylation and downregulation of MDM2 and MDM4 gene expression.
Chithra D Palani,James F Beck,Jürgen Sonnemann
Investigational new drugs
30
2011
Mostrar resumen
Nutlin-3, a small-molecule MDM2 inhibitor, restores p53 function and is, thus, an appealing candidate for the treatment of cancers retaining wild-type p53. However, nutlin-3 applied as single agent may be insufficient for cancer therapy. Therefore, we explored whether the anticancer activity of nutlin-3 could be enhanced by combination with histone deacetylase inhibitors (HDACi), i.e. vorinostat, sodium butyrate, MS-275 and apicidin. We found that nutlin-3 and HDACi cooperated to induce cell death in the p53 wild-type cell lines A549 and A2780, but not in the p53 null cell line PC-3, as assessed by Alamar Blue assay and flow cytometric analyses of propidium iodide uptake and mitochondrial depolarization. Combination index analysis showed that the effect was synergistic. For comparison, we tested nutlin-3 in combination with paclitaxel, revealing that nutlin-3 antagonized the cytotoxic activity of paclitaxel. To shed light on the underlying mechanism of the synergistic action of nutlin-3 and HDACi, we determined the acetylation status of p53 by immunoblotting and the mRNA levels of MDM2 and MDM4 by real-time RT-PCR. We observed vorinostat to induce p53 hyperacetylation, to reduce the constitutive gene expression of MDM2 and MDM4, and to counteract the nutlin-3-induced upregulation of MDM2 gene expression. In conclusion, our study shows that HDACi amplify the antitumor activity of nutlin-3-possibly by inducing p53 hyperacetylation and/or MDM2 and/or MDM4 downregulation-suggesting that treatment with a combination of nutlin-3 and HDACi may be an effective strategy for treating tumors with wild-type p53. | | 20680659
 |
Orphan nuclear receptor PNR/NR2E3 stimulates p53 functions by enhancing p53 acetylation.
Wen, Z; Pyeon, D; Wang, Y; Lambert, P; Xu, W; Ahlquist, P
Molecular and cellular biology
32
26-35
2011
Mostrar resumen
Since inactivation of tumor suppressor p53 functions is one of the most common features of human cancer cells, restoring p53 expression and activity is an important focus in cancer therapy. Here we report identification of photoreceptor-specific nuclear receptor (PNR)/NR2E3 as a positive regulator of p53 in a high-throughput genetic screen. In HeLa cells, PNR stimulated p53-responsive promoters in a p53-dependent fashion and induced apoptosis in several cell types. PNR also increased p53 protein stability and specific activity as a transcriptional activator. Our studies of the underlying mechanisms showed that PNR forms complexes with p53 and the acetyltransferase p300, stimulates p53 acetylation, and increases the expression of a subset of p53 target genes. Furthermore, PNR significantly boosted actinomycin D-stimulated p53 acetylation. The unique mechanisms by which PNR stimulates p53 acetylation and functions define this orphan nuclear receptor as a potentially valuable target and tool in p53-associated cancer therapy and offer new insights into the roles of PNR mutation in retinal diseases. | Western Blotting | 22025681
 |
Mechanisms of p53 activation and physiological relevance in the developing kidney.
Aboudehen, K; Hilliard, S; Saifudeen, Z; El-Dahr, SS
Am J Physiol Renal Physiol
302
F928-40
2011
Mostrar resumen
The tumor suppressor protein p53 is a short-lived transcription factor due to Mdm2-mediated proteosomal degradation. In response to genotoxic stress, p53 is stabilized via posttranslational modifications which prevent Mdm2 binding. p53 activation results in cell cycle arrest and apoptosis. We previously reported that tight regulation of p53 activity is an absolute requirement for normal nephron differentiation (Hilliard S, Aboudehen K, Yao X, El-Dahr SS Dev Biol 353: 354-366, 2011). However, the mechanisms of p53 activation in the developing kidney are unknown. We show here that metanephric p53 is phosphorylated and acetylated on key serine and lysine residues, respectively, in a temporal profile which correlates with the maturational changes in total p53 levels and DNA-binding activity. Site-directed mutagenesis revealed a differential role for these posttranslational modifications in mediating p53 stability and transcriptional regulation of renal function genes (RFGs). Section immunofluorescence also revealed that p53 modifications confer the protein with specific spatiotemporal expression patterns. For example, phos-p53(S392) is enriched in maturing proximal tubular epithelial cells, whereas acetyl-p53(K373/K382/K386) are expressed in nephron progenitors. Functionally, p53 occupancy of RFG promoters is enhanced at the onset of tubular differentiation, and p53 loss or gain of function indicates that p53 is necessary but not sufficient for RFG expression. We conclude that posttranslational modifications are important determinants of p53 stability and physiological functions in the developing kidney. We speculate that the stress/hypoxia of the embryonic microenvironment may provide the stimulus for p53 activation in the developing kidney. | Western Blotting | 22237799
 |
Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging.
Li, X; Khanna, A; Li, N; Wang, E
Aging
3
985-1002
2010
Mostrar resumen
MicroRNAs in blood samples have been identified as an important class of biomarkers, which can reflect physiological changes from cancer to brain dysfunction. In this report we identify concordant increases in levels of expression of miR-34a in brain and two components of mouse blood samples, peripheral blood mononuclear cells (PBMCs) and plasma, from 2 day old neonates through young adulthood and mid-life to old age at 25 months. Levels of this microRNA's prime target, silent information regulator 1 (SIRT1), in brain and the two blood-derived specimens decrease with age inversely to miR-34a, starting as early as 4 months old, when appreciable tissue aging has not yet begun. Our results suggest that: 1. Increased miR-34a and the reciprocal decrease of its target, SIRT1, in blood specimens are the accessible biomarkers for age-dependent changes in brain; and 2. these changes are predictors of impending decline in brain function, as early as in young adult mice. | | 22064828
 |
p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase.
Li, S; Banck, M; Mujtaba, S; Zhou, MM; Sugrue, MM; Walsh, MJ
PloS one
5
e10486
2009
Mostrar resumen
A hallmark of p53 function is to regulate a transcriptional program in response to extracellular and intracellular stress that directs cell cycle arrest, apoptosis, and cellular senescence. Independent of the role of p53 in the nucleus, some of the anti-proliferative functions of p53 reside within the mitochondria [1]. p53 can arrest cell growth in response to mitochondrial p53 in an EJ bladder carcinoma cell environment that is naïve of p53 function until induced to express p53 [2]. TP53 can independently partition with endogenous nuclear and mitochondrial proteins consistent with the ability of p53 to enact senescence. In order to address the role of p53 in navigating cellular senescence through the mitochondria, we identified SirT3 to rescue EJ/p53 cells from induced p53-mediated growth arrest. Human SirT3 function appears coupled with p53 early during the initiation of p53 expression in the mitochondria by biochemical and cellular localization analysis. Our evidence suggests that SirT3 partially abrogates p53 activity to enact growth arrest and senescence. Additionally, we identified the chaperone protein BAG-2 in averting SirT3 targeting of p53 -mediated senescence. These studies identify a complex relationship between p53, SirT3, and chaperoning factor BAG-2 that may link the salvaging and quality assurance of the p53 protein for control of cellular fate independent of transcriptional activity. | | 20463968
 |