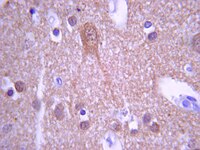
Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury.
Fisher, PW; Zhao, Y; Rico, MC; Massicotte, VS; Wade, CK; Litvin, J; Bove, GM; Popoff, SN; Barbe, MF
Journal of cell communication and signaling
9
37-54
2015
Mostrar resumen
Key clinical features of cumulative trauma disorders include pain, muscle weakness, and tissue fibrosis, although the etiology is still under investigation. Here, we characterized the temporal pattern of altered sensorimotor behaviors and inflammatory and fibrogenic processes occurring in forearm muscles and serum of young adult, female rats performing an operant, high repetition high force (HRHF) reaching and grasping task for 6, 12, or 18 weeks. Palmar mechanical sensitivity, cold temperature avoidance and spontaneous behavioral changes increased, while grip strength declined, in 18-week HRHF rats, compared to controls. Flexor digitorum muscles had increased MCP-1 levels after training and increased TNFalpha in 6-week HRHF rats. Serum had increased IL-1beta, IL-10 and IP-10 after training. Yet both muscle and serum inflammation resolved by week 18. In contrast, IFNγ increased at week 18 in both muscle and serum. Given the anti-fibrotic role of IFNγ, and to identify a mechanism for the continued grip strength losses and behavioral sensitivities, we evaluated the fibrogenic proteins CCN2, collagen type I and TGFB1, as well as the nociceptive/fibrogenic peptide substance P. Each increased in and around flexor digitorum muscles and extracellular matrix in the mid-forearm, and in nerves of the forepaw at 18 weeks. CCN2 was also increased in serum at week 18. At a time when inflammation had subsided, increases in fibrogenic proteins correlated with sensorimotor declines. Thus, muscle and nerve fibrosis may be critical components of chronic work-related musculoskeletal disorders. CCN2 and substance P may serve as potential targets for therapeutic intervention, and CCN2 as a serum biomarker of fibrosis progression. | | 25617052
 |
Downregulation of cannabinoid receptor 1 from neuropeptide Y interneurons in the basal ganglia of patients with Huntington's disease and mouse models.
Horne, EA; Coy, J; Swinney, K; Fung, S; Cherry, AE; Marrs, WR; Naydenov, AV; Lin, YH; Sun, X; Keene, CD; Grouzmann, E; Muchowski, P; Bates, GP; Mackie, K; Stella, N
The European journal of neuroscience
37
429-40
2013
Mostrar resumen
Cannabinoid receptor 1 (CB(1) receptor) controls several neuronal functions, including neurotransmitter release, synaptic plasticity, gene expression and neuronal viability. Downregulation of CB(1) expression in the basal ganglia of patients with Huntington's disease (HD) and animal models represents one of the earliest molecular events induced by mutant huntingtin (mHtt). This early disruption of neuronal CB(1) signaling is thought to contribute to HD symptoms and neurodegeneration. Here we determined whether CB(1) downregulation measured in patients with HD and mouse models was ubiquitous or restricted to specific striatal neuronal subpopulations. Using unbiased semi-quantitative immunohistochemistry, we confirmed previous studies showing that CB(1) expression is downregulated in medium spiny neurons of the indirect pathway, and found that CB(1) is also downregulated in neuropeptide Y (NPY)/neuronal nitric oxide synthase (nNOS)-expressing interneurons while remaining unchanged in parvalbumin- and calretinin-expressing interneurons. CB(1) downregulation in striatal NPY/nNOS-expressing interneurons occurs in R6/2 mice, Hdh(Q150/Q150) mice and the caudate nucleus of patients with HD. In R6/2 mice, CB(1) downregulation in NPY/nNOS-expressing interneurons correlates with diffuse expression of mHtt in the soma. This downregulation also occludes the ability of cannabinoid agonists to activate the pro-survival signaling molecule cAMP response element-binding protein in NPY/nNOS-expressing interneurons. Loss of CB(1) signaling in NPY/nNOS-expressing interneurons could contribute to the impairment of basal ganglia functions linked to HD. | | 23167744
 |
Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks.
Michaelides, M; Anderson, SA; Ananth, M; Smirnov, D; Thanos, PK; Neumaier, JF; Wang, GJ; Volkow, ND; Hurd, YL
The Journal of clinical investigation
123
5342-50
2013
Mostrar resumen
The ability to map the functional connectivity of discrete cell types in the intact mammalian brain during behavior is crucial for advancing our understanding of brain function in normal and disease states. We combined designer receptor exclusively activated by designer drug (DREADD) technology and behavioral imaging with μPET and [18F]fluorodeoxyglucose (FDG) to generate whole-brain metabolic maps of cell-specific functional circuits during the awake, freely moving state. We have termed this approach DREADD-assisted metabolic mapping (DREAMM) and documented its ability in rats to map whole-brain functional anatomy. We applied this strategy to evaluating changes in the brain associated with inhibition of prodynorphin-expressing (Pdyn-expressing) and of proenkephalin-expressing (Penk-expressing) medium spiny neurons (MSNs) of the nucleus accumbens shell (NAcSh), which have been implicated in neuropsychiatric disorders. DREAMM revealed discrete behavioral manifestations and concurrent engagement of distinct corticolimbic networks associated with dysregulation of Pdyn and Penk in MSNs of the NAcSh. Furthermore, distinct neuronal networks were recruited in awake versus anesthetized conditions. These data demonstrate that DREAMM is a highly sensitive, molecular, high-resolution quantitative imaging approach. | | 24231358
 |
The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders.
Barbe, MF; Gallagher, S; Massicotte, VS; Tytell, M; Popoff, SN; Barr-Gillespie, AE
BMC musculoskeletal disorders
14
303
2013
Mostrar resumen
We examined the relationship of musculoskeletal risk factors underlying force and repetition on tissue responses in an operant rat model of repetitive reaching and pulling, and if force x repetition interactions were present, indicative of a fatigue failure process. We examined exposure-dependent changes in biochemical, morphological and sensorimotor responses occurring with repeated performance of a handle-pulling task for 12 weeks at one of four repetition and force levels: 1) low repetition with low force, 2) high repetition with low force, 3) low repetition with high force, and 4) high repetition with high force (HRHF).Rats underwent initial training for 4-6 weeks, and then performed one of the tasks for 12 weeks, 2 hours/day, 3 days/week. Reflexive grip strength and sensitivity to touch were assayed as functional outcomes. Flexor digitorum muscles and tendons, forelimb bones, and serum were assayed using ELISA for indicators of inflammation, tissue stress and repair, and bone turnover. Histomorphometry was used to assay macrophage infiltration of tissues, spinal cord substance P changes, and tissue adaptative or degradative changes. MicroCT was used to assay bones for changes in bone quality.Several force x repetition interactions were observed for: muscle IL-1alpha and bone IL-1beta; serum TNFalpha, IL-1alpha, and IL-1beta; muscle HSP72, a tissue stress and repair protein; histomorphological evidence of tendon and cartilage degradation; serum biomarkers of bone degradation (CTXI) and bone formation (osteocalcin); and morphological evidence of bone adaptation versus resorption. In most cases, performance of the HRHF task induced the greatest tissue degenerative changes, while performance of moderate level tasks induced bone adaptation and a suggestion of muscle adaptation. Both high force tasks induced median nerve macrophage infiltration, spinal cord sensitization (increased substance P), grip strength declines and forepaw mechanical allodynia by task week 12.Although not consistent in all tissues, we found several significant interactions between the critical musculoskeletal risk factors of force and repetition, consistent with a fatigue failure process in musculoskeletal tissues. Prolonged performance of HRHF tasks exhibited significantly increased risk for musculoskeletal disorders, while performance of moderate level tasks exhibited adaptation to task demands. | | 24156755
 |
VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity.
Malykhina, AP; Lei, Q; Erickson, CS; Epstein, ML; Saban, MR; Davis, CA; Saban, R
BMC physiology
12
15
2011
Mostrar resumen
This work tests the hypothesis that bladder instillation with vascular endothelial growth factor (VEGF) modulates sensory and motor nerve plasticity, and, consequently, bladder function and visceral sensitivity.In addition to C57BL/6J, ChAT-cre mice were used for visualization of bladder cholinergic nerves. The direct effect of VEGF on the density of sensory nerves expressing the transient receptor potential vanilloid subfamily 1 (TRPV1) and cholinergic nerves (ChAT) was studied one week after one or two intravesical instillations of the growth factor.To study the effects of VEGF on bladder function, mice were intravesically instilled with VEGF and urodynamic evaluation was assessed. VEGF-induced alteration in bladder dorsal root ganglion (DRG) neurons was performed on retrogradly labeled urinary bladder afferents by patch-clamp recording of voltage gated Na+ currents. Determination of VEGF-induced changes in sensitivity to abdominal mechanostimulation was performed by application of von Frey filaments.In addition to an overwhelming increase in TRPV1 immunoreactivity, VEGF instillation resulted in an increase in ChAT-directed expression of a fluorescent protein in several layers of the urinary bladder. Intravesical VEGF caused a profound change in the function of the urinary bladder: acute VEGF (1 week post VEGF treatment) reduced micturition pressure and longer treatment (2 weeks post-VEGF instillation) caused a substantial reduction in inter-micturition interval. In addition, intravesical VEGF resulted in an up-regulation of voltage gated Na(+) channels (VGSC) in bladder DRG neurons and enhanced abdominal sensitivity to mechanical stimulation.For the first time, evidence is presented indicating that VEGF instillation into the mouse bladder promotes a significant increase in peripheral nerve density together with alterations in bladder function and visceral sensitivity. The VEGF pathway is being proposed as a key modulator of neural plasticity in the pelvis and enhanced VEGF content may be associated with visceral hyperalgesia, abdominal discomfort, and/or pelvic pain. | | 23249422
 |
Nerve growth factor derived from bronchial epithelium after chronic mite antigen exposure contributes to airway hyperresponsiveness by inducing hyperinnervation, and is inhibited by in vivo siRNA.
H Ogawa,M Azuma,H Uehara,T Takahashi,Y Nishioka,S Sone,K Izumi
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology
42
2011
Mostrar resumen
Bronchial asthma is a chronic allergic airway inflammatory disease. Neurotrophins, including nerve growth factor (NGF), play an important role in the pathogenesis of asthma. However, the effects of NGF derived from epithelium on airway hyperresponsiveness (AHR) after antigen sensitization/exposure remain uncertain. | | 22168511
 |
Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury.
Melanie B Elliott,Michael L Oshinsky,Peter S Amenta,Olatilewa O Awe,Jack I Jallo
Headache
52
2011
Mostrar resumen
This study tests the hypothesis that injury to the somatosensory cortex is associated with periorbital allodynia and increases in nociceptive neuropeptides in the brainstem in a mouse model of controlled cortical impact (CCI) injury. | | 22568499
 |
Three days after a single exposure to ozone, the mechanism of airway hyperreactivity is dependent on substance P and nerve growth factor.
Verhein, KC; Hazari, MS; Moulton, BC; Jacoby, IW; Jacoby, DB; Fryer, AD
American journal of physiology. Lung cellular and molecular physiology
300
L176-84
2010
Mostrar resumen
Ozone causes persistent airway hyperreactivity in humans and animals. One day after ozone exposure, airway hyperreactivity is mediated by release of eosinophil major basic protein that inhibits neuronal M(2) muscarinic receptors, resulting in increased acetylcholine release and increased smooth muscle contraction in guinea pigs. Three days after ozone, IL-1β, not eosinophils, mediates ozone-induced airway hyperreactivity, but the mechanism at this time point is largely unknown. IL-1β increases NGF and the tachykinin substance P, both of which are involved in neural plasticity. These experiments were designed to test whether there is a role for NGF and tachykinins in sustained airway hyperreactivity following a single ozone exposure. Guinea pigs were exposed to filtered air or ozone (2 parts per million, 4 h). In anesthetized and vagotomized animals, ozone potentiated vagally mediated airway hyperreactivity 24 h later, an effect that was sustained over 3 days. Pretreatment with antibody to NGF completely prevented ozone-induced airway hyperreactivity 3 days, but not 1 day, after ozone and significantly reduced the number of substance P-positive airway nerve bundles. Three days after ozone, NK(1) and NK(2) receptor antagonists also blocked this sustained hyperreactivity. Although the effect of inhibiting NK(2) receptors was independent of ozone, the NK(1) receptor antagonist selectively blocked vagal hyperreactivity 3 days after ozone. These data confirm mechanisms of ozone-induced airway hyperreactivity change over time and demonstrate 3 days after ozone that there is an NGF-mediated role for substance P, or another NK(1) receptor agonist, that enhances acetylcholine release and was not present 1 day after ozone. | | 21056958
 |
VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG.
Saban, MR; Davis, CA; Avelino, A; Cruz, F; Maier, J; Bjorling, DE; Sferra, TJ; Hurst, RE; Saban, R
BMC physiology
11
16
2010
Mostrar resumen
This work tests the hypothesis that increased levels of vascular endothelial growth factor (VEGF) observed during bladder inflammation modulates nerve plasticity.Chronic inflammation was induced by intravesical instillations of Bacillus Calmette-Guérin (BCG) into the urinary bladder and the density of nerves expressing the transient receptor potential vanilloid subfamily 1 (TRPV1) or pan-neuronal marker PGP9.5 was used to quantify alterations in peripheral nerve plasticity. Some mice were treated with B20, a VEGF neutralizing antibody to reduce the participation of VEGF. Additional mice were treated systemically with antibodies engineered to specifically block the binding of VEGF to NRP1 (anti-NRP1B) and NRP2 (NRP2B), or the binding of semaphorins to NRP1 (anti-NRP1 A) to diminish activity of axon guidance molecules such as neuropilins (NRPs) and semaphorins (SEMAs). To confirm that VEGF is capable of inducing inflammation and neuronal plasticity, another group of mice was instilled with recombinant VEGF165 or VEGF121 into the urinary bladder.The major finding of this work was that chronic BCG instillation resulted in inflammation and an overwhelming increase in both PGP9.5 and TRPV1 immunoreactivity, primarily in the sub-urothelium of the urinary bladder. Treatment of mice with anti-VEGF neutralizing antibody (B20) abolished the effect of BCG on inflammation and nerve density.NRP1A and NRP1B antibodies, known to reduce BCG-induced inflammation, failed to block BCG-induced increase in nerve fibers. However, the NRP2B antibody dramatically potentiated the effects of BCG in increasing PGP9.5-, TRPV1-, substance P (SP)-, and calcitonin gene-related peptide (CGRP)-immunoreactivity (IR). Finally, instillation of VEGF121 or VEGF165 into the mouse bladder recapitulated the effects of BCG and resulted in a significant inflammation and increase in nerve density.For the first time, evidence is being presented supporting that chronic BCG instillation into the mouse bladder promotes a significant increase in peripheral nerve density that was mimicked by VEGF instillation. Effects of BCG were abolished by pre-treatment with neutralizing VEGF antibody. The present results implicate the VEGF pathway as a key modulator of inflammation and nerve plasticity, introduces a new animal model for investigation of VEGF-induced nerve plasticity, and suggests putative mechanisms underlying this phenomenon. | | 22059553
 |
Morphological changes in the enteric nervous system of aging and APP23 transgenic mice.
Van Ginneken C, Schäfer KH, Van Dam D, Huygelen V, De Deyn PP
Brain Res
1378
43-53. Epub 2011 Jan 15.
2010
Mostrar resumen
Gastrointestinal motility disorders often pose a debilitating problem, especially in elderly patients. In addition, they are frequently occurring co-morbidities in dementia. Whereas a failing enteric nervous system has already been shown to be involved in gastrointestinal motility disorders and in Parkinson\'s disease, a relationship with the neurodegenerative process of Alzheimer\'s disease was not yet shown. Therefore, we sought to document quantitative changes in the distribution of βIII-tubulin (general neuronal marker), Substance P, neuronal nitric oxide synthase (NOS), glial fibrillary acidic protein (GFAP) and S-100 immunoreactivity in addition to a qualitative assessment of the presence of amyloid in the small and large intestines of 6, 12 and 18-month-old wild type and transgenic Thy-1-APP23 mice. Amyloid deposits were seen in the vasculature, the mucosal and muscle layer of both heterozygous and wild type mice. Amyloidβ₁₋₄₂ could not be detected, pointing to a different amyloid composition than that found in senile plaques in the mice\'s brains. The finding of an increased density of βIII-tubulin-, Substance P- and NOS-IR-nerve fibres in heterozygous mice could not undoubtedly be related to amyloid deposition or to an activation of glial cells. Therefore, the alterations at the level of the enteric nervous system and the deposition of amyloid seem not primarily involved in the pathogenesis of Alzheimer\'s disease. At most they are secondary related to the neurodegenerative process. Additionally, our data could not show extensive neuronal or glial cell loss associated with aging, in contrast to other reports. Instead an increase in S100-IR was observed in senescent mice.Copyright © 2011 Elsevier B.V. All rights reserved. | | 21241669
 |