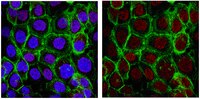
Sustained Pax6 Expression Generates Primate-like Basal Radial Glia in Developing Mouse Neocortex.
Wong, FK; Fei, JF; Mora-Bermúdez, F; Taverna, E; Haffner, C; Fu, J; Anastassiadis, K; Stewart, AF; Huttner, WB
PLoS biology
13
e1002217
2015
Mostrar resumen
The evolutionary expansion of the neocortex in mammals has been linked to enlargement of the subventricular zone (SVZ) and increased proliferative capacity of basal progenitors (BPs), notably basal radial glia (bRG). The transcription factor Pax6 is known to be highly expressed in primate, but not mouse, BPs. Here, we demonstrate that sustaining Pax6 expression selectively in BP-genic apical radial glia (aRG) and their BP progeny of embryonic mouse neocortex suffices to induce primate-like progenitor behaviour. Specifically, we conditionally expressed Pax6 by in utero electroporation using a novel, Tis21-CreERT2 mouse line. This expression altered aRG cleavage plane orientation to promote bRG generation, increased cell-cycle re-entry of BPs, and ultimately increased upper-layer neuron production. Upper-layer neuron production was also increased in double-transgenic mouse embryos with sustained Pax6 expression in the neurogenic lineage. Strikingly, increased BPs existed not only in the SVZ but also in the intermediate zone of the neocortex of these double-transgenic mouse embryos. In mutant mouse embryos lacking functional Pax6, the proportion of bRG among BPs was reduced. Our data identify specific Pax6 effects in BPs and imply that sustaining this Pax6 function in BPs could be a key aspect of SVZ enlargement and, consequently, the evolutionary expansion of the neocortex. | | | 26252244
 |
Combination of 12-O-tetradecanoylphorbol-13-acetate with diethyldithiocarbamate markedly inhibits pancreatic cancer cell growth in 3D culture and in immunodeficient mice.
Huang, H; Cao, K; Malik, S; Zhang, Q; Li, D; Chang, R; Wang, H; Lin, W; Van Doren, J; Zhang, K; Du, Z; Zheng, X
International journal of molecular medicine
35
1617-24
2015
Mostrar resumen
The aim of the present study was to determine the effects of 12-O-tetradecanoylphorbol-13-acetate (TPA) and diethyldithiocarbamate (DDTC) alone or in combination on human pancreatic cancer cells cultured in vitro and grown as xenograft tumors in nude mice. Pancreatic cancer cells were treated with either DDTC or TPA alone, or in combination and the number of viable cells was then determined by trypan blue ecxlusion assay and the number of apoptotic cells was determined by morphological assessment by staining the cells with propidium idiode and examining them under a fluorescence microscope. Treatment with DDTC or TPA alone inhibited the growth and promoted the apoptosis of pancreatic cancer cells in a concentration-dependent manner. These effects were more prominent following treatment with TPA in combination with DDTC than following treatment with either agent alone in PANC-1 cells in monolayer cultures and in 3 dimensional (3D) cultures. The potent effects of the combination treatment on PANC-1 cells were associated with the inhibition of nuclear factor-κB (NF-κB) activation and the decreased expression of Bcl-2 induced by DDTC, as shown by NF-κB-dependent reporter gene expression assay and western blot analysis. Furthermore, treatment of nude mice with DDTC + TPA strongly inhibited the growth of PANC-1 xenograft tumors. The results of the present study indicate that the administration of TPA and DDTC in combination may be an effective strategy for inhibiting the growth of pancreatic cancer. | | | 25847449
 |
A novel model of surgical injury in adult rat kidney: a "pouch model".
Litbarg, NO; Vujicic, S; Setty, S; Sethupathi, P; Dunea, G; Arruda, JA; Singh, AK
Scientific reports
3
2890
2013
Mostrar resumen
Regenerative mechanisms after surgical injury have been studied in many organs but not in the kidney. Studying surgical injury may provide new insights into mechanisms of kidney regeneration. In rodent models, extrarenal tissues adhere to surgical kidney wound and interfere with healing. We hypothesized that this can be prevented by wrapping injured kidney in a plastic pouch. Adult rats tolerated 5/6 nephrectomy with pouch application well. Histological analysis demonstrates that application of the pouch effectively prevented formation of adhesions and induced characteristic wound healing manifested by formation of granulation tissue. Additionally, selected tubules of the wounded kidney extended into the granulation tissue forming branching tubular epithelial outgrowths (TEOs) without terminal differentiation. Tubular regeneration outside of renal parenchyma was not previously observed, and suggests previously unrecognized capacity for regeneration. Our model provides a novel approach to study kidney wound healing. | | | 24100472
 |
Stepwise activation of the ATR signaling pathway upon increasing replication stress impacts fragile site integrity.
Koundrioukoff, S; Carignon, S; Técher, H; Letessier, A; Brison, O; Debatisse, M
PLoS genetics
9
e1003643
2013
Mostrar resumen
Breaks at common fragile sites (CFS) are a recognized source of genome instability in pre-neoplastic lesions, but how such checkpoint-proficient cells escape surveillance and continue cycling is unknown. Here we show, in lymphocytes and fibroblasts, that moderate replication stresses like those inducing breaks at CFSs trigger chromatin loading of sensors and mediators of the ATR pathway but fail to activate Chk1 or p53. Consistently, we found that cells depleted of ATR, but not of Chk1, accumulate single-stranded DNA upon Mre11-dependent resection of collapsed forks. Partial activation of the pathway under moderate stress thus takes steps against fork disassembly but tolerates S-phase progression and mitotic onset. We show that fork protection by ATR is crucial to CFS integrity, specifically in the cell type where a given site displays paucity in backup replication origins. Tolerance to mitotic entry with under-replicated CFSs therefore results in chromosome breaks, providing a pool of cells committed to further instability. | | | 23874235
 |
Phenothiazine Inhibitors of TLKs Affect Double-Strand Break Repair and DNA Damage Response Recovery and Potentiate Tumor Killing with Radiomimetic Therapy.
Ronald, S; Awate, S; Rath, A; Carroll, J; Galiano, F; Dwyer, D; Kleiner-Hancock, H; Mathis, JM; Vigod, S; De Benedetti, A
Genes & cancer
4
39-53
2013
Mostrar resumen
The Tousled-like kinases (TLKs) are involved in chromatin assembly, DNA repair, and transcription. Two TLK genes exist in humans, and their expression is often dysregulated in cancer. TLKs phosphorylate Asf1 and Rad9, regulating double-strand break (DSB) repair and the DNA damage response (DDR). TLKs maintain genomic stability and are important therapeutic intervention targets. We identified specific inhibitors of TLKs from several compound libraries, some of which belong to the family of phenothiazine antipsychotics. The inhibitors prevented the TLK-mediated phosphorylation of Rad9(S328) and impaired checkpoint recovery and DSB repair. The inhibitor thioridazine (THD) potentiated tumor killing with chemotherapy and also had activity alone. Staining for γ-H2AX revealed few positive cells in untreated tumors, but large numbers in mice treated with low doxorubicin or THD alone, possibly the result of the accumulation of DSBs that are not promptly repaired as they may occur in the harsh tumor growth environment. | | | 23946870
 |
Rnf165/Ark2C enhances BMP-Smad signaling to mediate motor axon extension.
Kelly, CE; Thymiakou, E; Dixon, JE; Tanaka, S; Godwin, J; Episkopou, V
PLoS biology
11
e1001538
2013
Mostrar resumen
Little is known about extrinsic signals required for the advancement of motor neuron (MN) axons, which extend over long distances in the periphery to form precise connections with target muscles. Here we present that Rnf165 (Arkadia-like; Arkadia2; Ark2C) is expressed specifically in the nervous system and that its loss in mice causes motor innervation defects that originate during development and lead to wasting and death before weaning. The defects range from severe reduction of motor axon extension as observed in the dorsal forelimb to shortening of presynaptic branches of the phrenic nerve, as observed in the diaphragm. Molecular functional analysis showed that in the context of the spinal cord Ark2C enhances transcriptional responses of the Smad1/5/8 effectors, which are activated (phosphorylated) downstream of Bone Morphogenetic Protein (BMP) signals. Consistent with Ark2C-modulated BMP signaling influencing motor axons, motor pools in the spinal cord were found to harbor phosphorylated Smad1/5/8 (pSmad) and treatment of primary MN with BMP inhibitor diminished axon length. In addition, genetic reduction of BMP-Smad signaling in Ark2C (+/-) mice caused the emergence of Ark2C (-/-) -like dorsal forelimb innervation deficits confirming that enhancement of BMP-Smad responses by Ark2C mediates efficient innervation. Together the above data reveal an involvement of BMP-Smad signaling in motor axon advancement. | | | 23610558
 |
Assessment of adult neurogenesis in mice.
Pan, YW; Wang, W; Xia, Z
Current protocols in toxicology / editorial board, Mahin D. Maines (editor-in-chief) ... [et al.]
Chapter 12
Unit12.20
2013
Mostrar resumen
Adult neurogenesis is a lifelong developmental process that occurs in two discrete regions in the adult mammalian brain: the subgranular zone of the dentate gyrus (DG) and the subventricular zone (SVZ) along the lateral ventricles. Despite immense interest in the therapeutic potential of adult neural stem cells (aNSCs) residing along these two neurogenic regions, molecular and cellular mechanisms regulating this process are not fully defined. Defining the regulatory mechanisms responsible for the genesis of new neurons in the adult brain is integral to understanding the basic biology of aNSCs. The techniques described here provide a basic blueprint to isolate, culture, and perform experiments using aNSCs in vitro as well as providing methods to perform immunohistochemistry on brain sections. Curr. Protoc. Toxicol. 56:12.20.1-12.20.16. © 2013 by John Wiley & Sons, Inc. | | | 23670864
 |
Cellular host responses to gliomas.
Najbauer, J; Huszthy, PC; Barish, ME; Garcia, E; Metz, MZ; Myers, SM; Gutova, M; Frank, RT; Miletic, H; Kendall, SE; Glackin, CA; Bjerkvig, R; Aboody, KS
PloS one
7
e35150
2011
Mostrar resumen
Glioblastoma multiforme (GBM) is the most aggressive type of malignant primary brain tumors in adults. Molecular and genetic analysis has advanced our understanding of glioma biology, however mapping the cellular composition of the tumor microenvironment is crucial for understanding the pathology of this dreaded brain cancer. In this study we identified major cell populations attracted by glioma using orthotopic rodent models of human glioma xenografts. Marker-specific, anatomical and morphological analyses revealed a robust influx of host cells into the main tumor bed and tumor satellites.Human glioma cell lines and glioma spheroid orthotopic implants were used in rodents. In both models, the xenografts recruited large numbers of host nestin-expressing cells, which formed a 'network' with glioma. The host nestin-expressing cells appeared to originate in the subventricular zone ipsilateral to the tumor, and were clearly distinguishable from pericytes that expressed smooth muscle actin. These distinct cell populations established close physical contact in a 'pair-wise' manner and migrated together to the deeper layers of tumor satellites and gave rise to tumor vasculature. The GBM biopsy xenografts displayed two different phenotypes: (a) low-generation tumors (first in vivo passage in rats) were highly invasive and non-angiogenic, and host nestin-positive cells that infiltrated into these tumors displayed astrocytic or elongated bipolar morphology; (b) high-generation xenografts (fifth passage) had pronounced cellularity, were angiogenic with 'glomerulus-like' microvascular proliferations that contained host nestin-positive cells. Stromal cell-derived factor-1 and its receptor CXCR4 were highly expressed in and around glioma xenografts, suggesting their role in glioma progression and invasion.Our data demonstrate a robust migration of nestin-expressing host cells to glioma, which together with pericytes give rise to tumor vasculature. Mapping the cellular composition of glioma microenvironment and deciphering the complex 'crosstalk' between tumor and host may ultimately aid the development of novel anti-glioma therapies. | Immunohistochemistry (Paraffin) | | 22539956
 |
Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus.
Kelava, I; Reillo, I; Murayama, AY; Kalinka, AT; Stenzel, D; Tomancak, P; Matsuzaki, F; Lebrand, C; Sasaki, E; Schwamborn, JC; Okano, H; Huttner, WB; Borrell, V
Cerebral cortex (New York, N.Y. : 1991)
22
469-81
2011
Mostrar resumen
Subventricular zone (SVZ) progenitors are a hallmark of the developing neocortex. Recent studies described a novel type of SVZ progenitor that retains a basal process at mitosis, sustains expression of radial glial markers, and is capable of self-renewal. These progenitors, referred to here as basal radial glia (bRG), occur at high relative abundance in the SVZ of gyrencephalic primates (human) and nonprimates (ferret) but not lissencephalic rodents (mouse). Here, we analyzed the occurrence of bRG cells in the embryonic neocortex of the common marmoset Callithrix jacchus, a near-lissencephalic primate. bRG cells, expressing Pax6, Sox2 (but not Tbr2), glutamate aspartate transporter, and glial fibrillary acidic protein and retaining a basal process at mitosis, occur at similar relative abundance in the marmoset SVZ as in human and ferret. The proportion of progenitors in M-phase was lower in embryonic marmoset than developing ferret neocortex, raising the possibility of a longer cell cycle. Fitting the gyrification indices of 26 anthropoid species to an evolutionary model suggested that the marmoset evolved from a gyrencephalic ancestor. Our results suggest that a high relative abundance of bRG cells may be necessary, but is not sufficient, for gyrencephaly and that the marmoset's lissencephaly evolved secondarily by changing progenitor parameters other than progenitor type. | Immunofluorescence | | 22114084
 |
Inhibition of HIV-1 Tat-mediated transcription by a coumarin derivative, BPRHIV001, through the Akt pathway.
Lin, PH; Ke, YY; Su, CT; Shiao, HY; Hsieh, HP; Chao, YK; Lee, CN; Kao, CL; Chao, YS; Chang, SY
Journal of virology
85
9114-26
2010
Mostrar resumen
The human immunodeficiency virus type 1 (HIV-1)-encoded RNA-binding protein Tat is known to play an essential role in viral gene expression. In the search for novel compounds to inhibit Tat transactivity, one coumarin derivative, BPRHIV001, was identified, with a 50% effective concentration (EC(50)) against HIV-1 at 1.3 nM. BPRHIV001 is likely to exert its effects at the stage after initiation of RNAPII elongation since Tat protein expression and the assembly of the Tat/P-TEFb complex remained unchanged. Next, a reduction of the p300 protein level, known to modulate Tat function through acetylation, was observed upon BPRHIV001 treatment, while the p300 mRNA level was unaffected. A concordant reduction of phosphorylated Akt, which was shown to be closely related to p300 stability, was observed in the presence of BPRHIV001 and was accompanied by a decrease of phosphorylated PDPK1, a well-known Akt activator. Furthermore, the docking analysis revealed that the reduced PDPK1 phosphorylation likely resulted from the allosteric effect of interaction between BPRHIV001 and PDPK1. With strong synergistic effects with current reverse transcriptase inhibitors, BPRHIV001 has the potential to become a promising lead compound for the development of a novel therapeutic agent against HIV-1 infection. | | | 21697490
 |